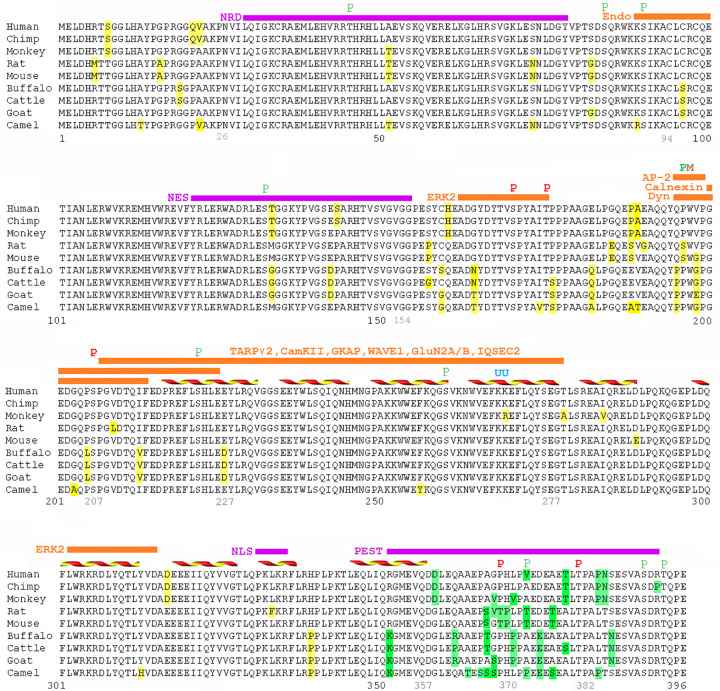
Fig 1. Sequence alignment of Arc proteins in different mammal species.
The sequence of Arc proteins in different mammal species were aligned and compared with each other. The non-conserved amino-acid residues in the protein sequences were highlighted in green color (in the C-terminal sequence segment Arc351-396) or yellow color (in the rest of the protein). The alignment result shows that the amino-acid residue composition in the C-terminal tail region of the Arc protein (~ Arc351-396) is highly divergent compared to the rest of the protein. The purple bars above the protein sequence indicate the sequence regions that are important for Arc’s nuclear transportation (NRD, NES, NLS) [14] and degradation (PEST) [28]; the orange bars indicate the sequence regions that are important for Arc’s interaction with other proteins (these proteins’ names were labeled before/above the orange bars) [10, 11, 13, 15, 20, 29–33]; the colored letters indicate Arc’s phosphorylation sites (red “P”) [31, 34], putative phosphorylation sites (green “P”) [1, 2], ubiquitination sites (blue “U”) [13], and mutation site (brown “M”) [30]; the yellow-red ribbons indicate the α-helix secondary structures in Arc’s capsid domain [15, 20]. (See the Discussion section for more detail).