Figure 9.
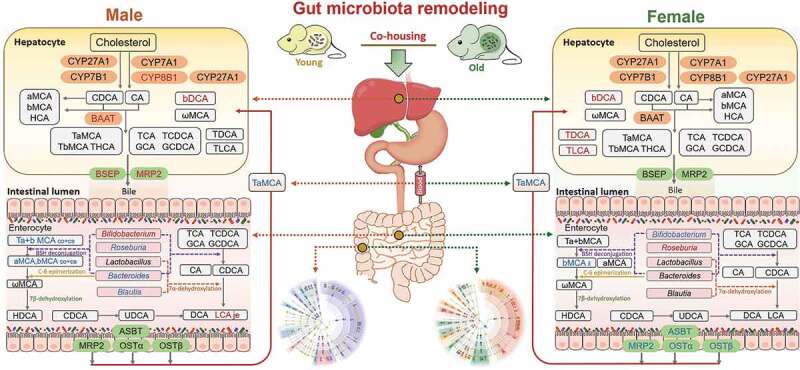
Gut microbiota remodeling by co-housing reverses the dysregulation of systemic BA homeostasis induced by aging.
Old mice of both sexes had dramatic changes in gut microbiota composition and BA profiles compared with their young counterparts. To investigate the impact of gut microbiota composition on aging-associated disorders and bile acids metabolism, we performed a co-housing experiment within the same sex. After co-housing, the host phenotypes were reversed, and the microbiota profile in co-housed old mice was shifted. Next, we investigated the expression of genes involved in BA biosynthesis, transport, and metabolism.12,13,45,54 In the liver, co-housing increased Cyp8b1, Baat, Bsep, and Mrp2 levels in old male mice, while there was no significant change in old female mice. The concentrations of hepatic βDCA were increased after co-housing in both male and female mice, while TLCA and TDCA were only increased after co-housing in female mice. In the ileum, decreased trends of Asbt, Mrp2, Ostα, and Ostβ were observed in co-housed old female mice, while expression of these genes did not show marked changes after co-housing in male mice. Meanwhile, Bifidobacterium was increased and reduced in co-housed old male and female mice, respectively. In addition, Roseburia, Blautia, and Bacteroides were reduced in co-housed old male mice, while these genera did not show consistent changes after co-housing in female mice. Moreover, the intestinal concentrations of individual BAs were divergently altered upon co-housing in the jejunum (je), ileum (il), cecum (ce), and colon (co) in a sex-dependent manner. Furthermore, in the serum, TαMCA was reduced in old male and female mice upon co-housing. In summary, co-housing reversed aging-associated dysregulation of systemic bile acid homeostasis in mice in a sex-dependent manner. Red and blue represent increased and decreased gene expression and BAs levels in co-housed old mice when compared with old mice, respectively.
