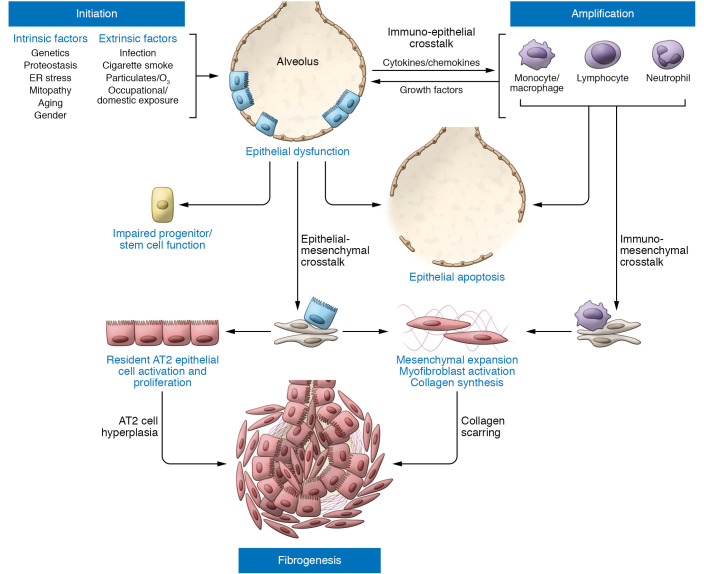
Figure 1. IPF pathogenesis driven by epithelial dysfunction occurs in three phases.
Initiation: Intrinsic (e.g., genetic) and extrinsic (e.g., infection, air pollution) factors acting through various pathways converge to produce a vulnerable alveolar type 2 epithelial cell (AT2) population (blue rectangular cells). Vulnerable AT2s subjected to continued intrinsic proteostatic/cell quality control challenges or additional secondary injurious stimuli (often recurrent) develop profound functional defects marked by aberrant activation of developmental programs, enhanced cell stress responses, impaired progenitor function, and/or apoptosis. Amplification: Dysfunctional AT2 cells (red rectangular cells) can initiate crosstalk with immune populations such as Ly6Chi monocytes, alveolar macrophages, neutrophils, or lymphocytes, which can both amplify the initial injury events and promote mesenchymal expansion further complemented by commensurate AT2/mesenchymal crosstalk. Fibrogenesis: The dysfunctional alveolar niche exhibits further feed-forward mechanisms to promote ongoing AT2 dysfunction marked by increased proliferation (AT2 hyperplasia), impaired transdifferentiation to AT1 cells, and upregulation of senescence programs. Coupled with enhanced myofibroblast activation and matrix deposition, the disrupted injury/repair response leads to scar formation and progressive loss of lung architectural complexity culminating in progressive fibrotic remodeling, physiological derangements in gas exchange, and a clinically evident IPF phenotype.