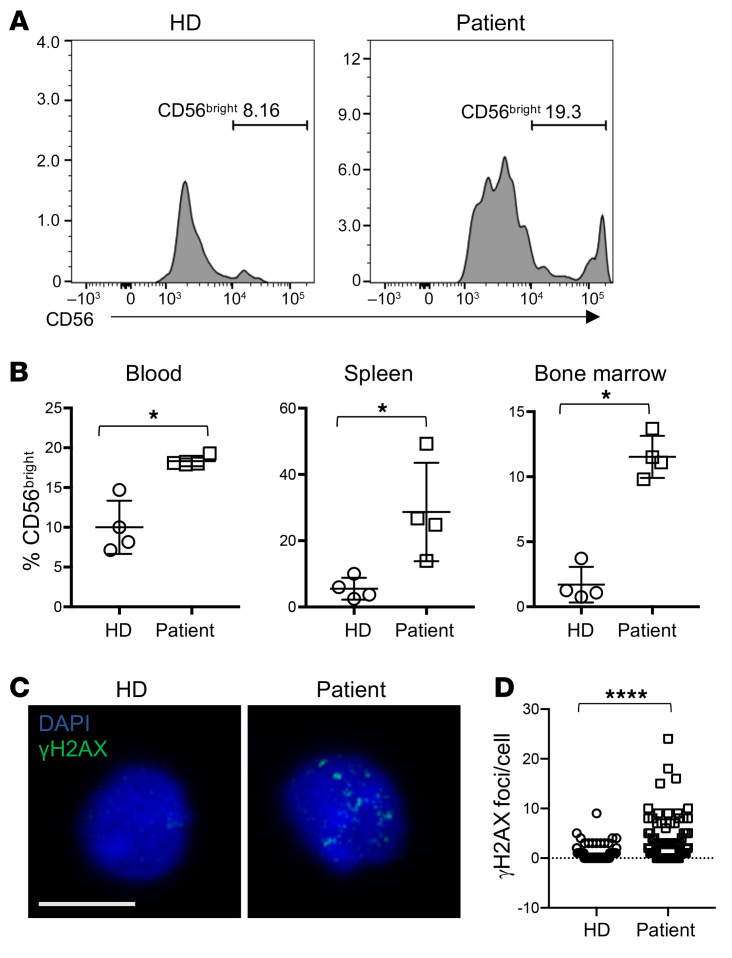
Figure 9. iPS cell–derived NK cells from the proband have impaired terminal maturation and increased replication stress.
iPS cells reprogrammed from patient primary fibroblasts were differentiated by teratoma formation to CD34+ HSCs, purified, and transplanted into NSG mice as described in Methods. Organs were harvested 21 days following transplantation, and human CD45+CD56+CD3− cells were analyzed for density of CD56 expression. n = 4 mice per genotype (patient and healthy donor–derived iPS cells). (A) Representative FACS histograms of NK cells from bone marrow of mice reconstituted with human NK cells from healthy donor– or patient-derived CD34+ cells generated from iPS cells. (B) Frequency of CD56bright NK cells from 4 mice per genotype from blood, spleen, and bone marrow, as indicated. NK cells were identified as human CD45+CD56+CD3− (bone marrow: 93–979 NK cells; blood: 20–405 NK cells; spleen: 60–880 NK cells, all from >106 cells from each organ per mouse), and the frequency of CD56bright NK cells based on CD56 density within the human NK cell population is shown. *P < 0.05, Mann-Whitney U test. (C) Splenocytes from mice transplanted with HD or patient-specific iPS cell–derived CD34+ cells were fixed, permeabilized, and incubated with anti-γH2AX antibody. Images were acquired by confocal microscopy. Scale bar: 5 μm. (D) Frequency of γH2AX foci per cell were enumerated by manual counting of 109 (HD) and 119 (patient) cells. ****P < 0.0001, Mann-Whitney U test. Data were pooled from 4 mice per genotype and are represented as mean ± 95% CI.