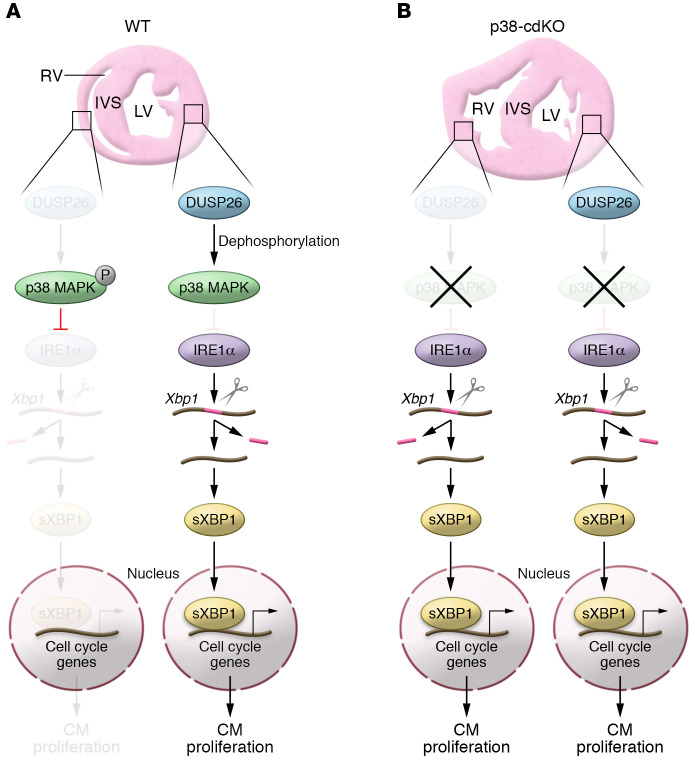
Figure 1. Model of DUSP26/p38 MAPK/IRE1α/XBP1 pathway in controlling cardiomyocyte (CM) proliferation in ventricles.
(A) In the WT neonatal heart, low DUSP26 levels in the RV are insufficient to dephosphorylate p38 MAPK, which in turn represses the expression of IRE1α and shuts down the IRE1α/XBP1 pathway, leading to lower CM proliferation in the RV. In contrast, LV-enriched DUSP26 dephosphorylates p38 MAPK, thus relieving p38 MAPK’s inhibitory effect on the expression of IRE1α, which splices Xbp1 mRNA to generate the sXbp1 isoform. sXBP1 then translocates to the nucleus and drives the expression of cell cycle–related genes, promoting CM proliferation. (B) In the p38-cdKO heart, the expression of IRE1α in the RV is no longer repressed by p38 MAPK, resulting in ectopic activation of the IRE1α/XBP1 pathway, which ultimately leads to excessive RV growth and dysfunction. In contrast, the p38-cdKO LV is unaffected because p38 MAPK activity is already very low in the WT LV.