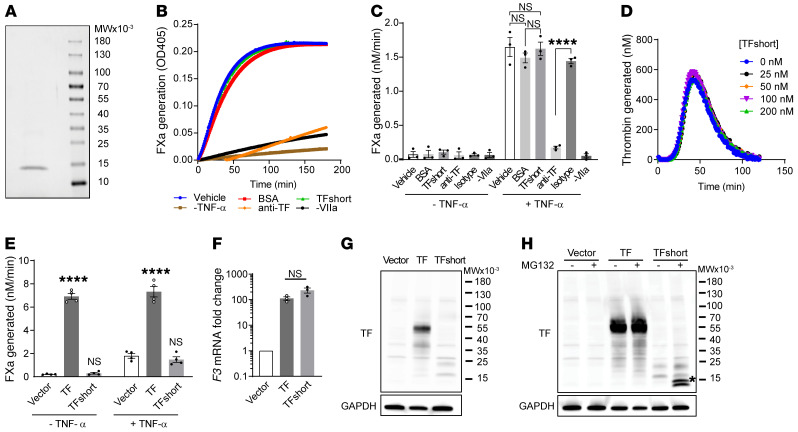
Figure 2. Truncated TF neither enhances nor inhibits coagulant activity.
(A) Purified recombinant TFshort encoding p.Ser117HisfsTer10 visualized by Coomassie stain migrates as a single band following SDS-PAGE. (B) TFshort (30 nM) was evaluated for its influence on the TF/factor VIIa catalyzed conversion of factor X to factor Xa on the surface of TNF-α stimulated HUVECs, as determined by cleavage of a chromogenic substrate monitoring factor Xa activity. Controls include treatment with bovine serum albumin (BSA), a TF inhibitory antibody (anti-TF) or its isotype control, or omission of TNF-α or factor VIIa. A representative kinetic course is shown, with error bars depicting SEM for 3 replicates. (C) Quantification of B across n = 3 independent experiments; 1-way analysis of variance, ****P < 0.0001. (D) The kinetics of thrombin generation initiated by lipidated TF in the presence of ascending TFshort concentrations, as indicated. (E) HUVECs were transduced with lentivirus encoding TF, TFshort, or vector control and cells were analyzed for their ability to support factor Xa generation with or without TNF-α stimulation; n = 4, 1-way analysis of variance, ****P < 0.0001. (F) HUVECs were transduced as in E and F3 mRNA was analyzed via quantitative PCR; n = 3, 1-way analysis of variance, not significant. (G) As in F, but TF expression was determined by immunoblot with anti-TF and anti-GAPDH antibodies. (H) As in G, but cells were treated with 100 μM MG-132 or vehicle for 6 hours before analysis; asterisk highlights the stabilized fragment. See complete unedited blots in the supplemental material.