Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an acquired immune-mediated disease of the peripheral nervous system, either being isolated or associated with other systemic diseases, most frequently with lymphoproliferative subtypes.1 CIDP concurrent with monoclonal gammopathy of undetermined significance (MGUS) accounts for up to 20%–30% of all cases,2 is often considered clinically indistinguishable from typical CIDP, and responds similarly to immunologic therapies.3 CIDP clinical course may be poorly responsive to conventional immunologic or chemotherapy treatment.4 Consequently, allogeneic and autologous hematopoietic stem cell transplantations (HSCTs) have been proposed in selected cases.5 To our knowledge, only a single patient with concurrent IgG lambda MGUS has been treated with autologous HSCT,6 and no case of IgG kappa MGUS is reported so far.
Clinical case
A previously healthy 49-year-old male patient presented with a 4-week history of progressive muscle weakness, gait ataxia, perioral paresthesias, and subjective swallowing difficulties. Neurologic examination revealed normal cranial nerves—proximal rather than distal paresis with diffusely absent reflexes and mild lower limb sensory deficiencies. CSF analysis showed increased protein (1,185 mg/L) and normal cell count. Electrophysiologic nerve conduction studies disclosed findings of demyelinating polyneuropathy (table e-1, links.lww.com/NXI/A313); needle electromyographic examination in the arms and legs was normal. Serum VEGF concentration was also normal (134 pg/mL). Nerve ultrasound and spinal MRI showed diffuse hypertrophic, hyperechogenic nerves (figure e-1, links.lww.com/NXI/A312).
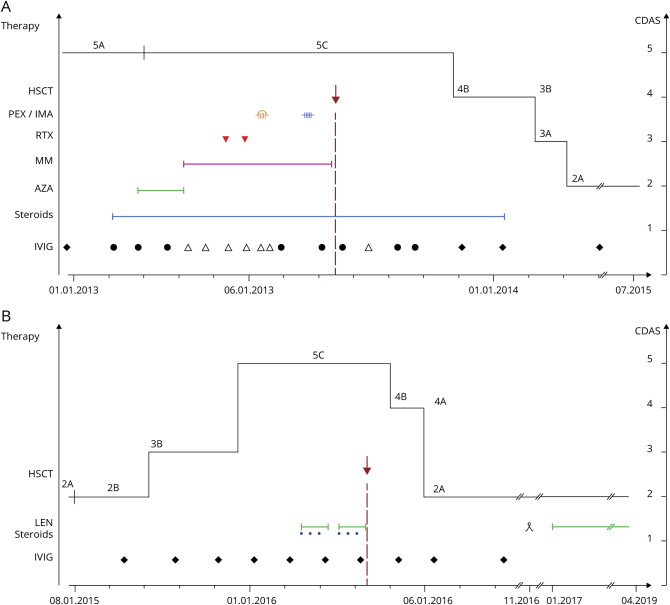
Figure 1 illustrates the clinical course and therapy schedule.
Figure. Clinical course of neurologic symptoms according to CDAS and treatment strategies at presentation and at relapse.
(A) Timeline and relationship between neurologic symptoms according to CDAS and treatment strategies at presentation. CDAS score 5 represent unstable active disease with abnormal examination and progressive course (5A treatment <3 months, 5C on treatment). Score 4: improvement >3 months, <1 year (4A with normal examination, 4B with abnormal examination). Score 3: stable active disease >1 year (3A with normal examination, 3B with abnormal examination). Score 2: remission <5 years, off treatment (2A with normal examination, 2B with abnormal examination). Score 1 cure >5 years, off treatment. (B) Timeline and relationship between treatment and neurologic symptoms according to CDAS at relapse. IVIG, high-dose immunoglobulin: symbol represent IVIG treatment cycles: ● 0.4 g/kg (35 g), ∆ 1.0 g/kg (85 g), ♦ 2 g/kg (160 g); blue line: steroids; green line: AZA; purple line: MM; red triangle (▼): RTX; orange: PEX; blue: IMA; green line fine: LEN; red arrow (↓): autologous HSCT; ᴥ preventive hematopoietic stem cell mobilization and collection. AZA = azathioprine; CDAS = CIDP disease activity status; HSCT = hematopoietic stem cell transplantation; IMA = immunoabsorption; IVIG = IV immunoglobulin; LEN = lenalidomide; MM = mycophenolate mofetil; PEX = plasmapheresis; RTX = rituximab.
CIDP was diagnosed according to European Federation of Neurological Societies/Peripheral Nerve Society criteria,7 although a sural nerve biopsy was not deemed necessary.
Bone marrow aspirate smears and trephine biopsy demonstrated IgG kappa MGUS with 4% kappa light chain–restricted plasma cells (table e-2, links.lww.com/NXI/A314). There was no evidence of amyloidosis or other hematologic malignancies on whole body CT scan and bone marrow biopsy. Fluorescence in-situ hybridization (FISH) revealed no cytogenetic abnormalities, and CRAB criteria (elevated calcium level, renal failure, anemia, and bone lesions) were not met.
In July 2013, walking distance was less than 20 m with a cane, and the patient developed respiratory distress necessitating mechanical respiratory support. High-dose chemotherapy with melphalan, followed by autologous HSCT together with continued IV immunoglobulin (IVIG) therapy were administered as salvage therapy (figure 1).
Conditioning treatment was melphalan (200 mg/m2 IV). Stem cell mobilization for autologous HSCTs was performed by vinorelbine (35 mg/m2 IV) and G-CSF (10 μg/kg body weight). A total of 3.2 × 106 CD34+ cells/kg b.w. were transfused at the day 0 of autologous HSCT. The patient fully recovered and resumed his professional activities (figure 1). The free-serum light chain ratio and the previously suppressed lambda light chains normalized (table e-2, links.lww.com/NXI/A314).
Two years after HSCT, the patient presented with a clinical relapse. Bone marrow analysis was again compatible with IgG kappa MGUS with 4% kappa light-chain restricted plasma cells (table e-2, links.lww.com/NXI/A314). Chromosome banding analysis and FISH at that time identified monosomy 13q14.3 and t (11;14). Despite IVIG, the neurologic symptoms worsened, and a second autologous HSCT was performed after conditioning with melphalan (200 mg/m2 IV) preceded by 2 cycles of lenalidomide (25 mg/d on a 21‐days cycle) and steroids. Neurologic symptoms completely resolved within weeks (figure 1). Continuous maintenance therapy with lenalidomide 10 mg/d on 21‐days cycles was started. Three years after the second HSCT, the patient was still asymptomatic.
Discussion
We describe a strong and timely correlation between 2 HSCTs and the resolution of the CIDP-related neurologic symptoms. Particularly at relapse, when the second HSCT was performed immediately after clinical aggravation, clinical improvement was prompt. Neurologic improvement was closely correlated with the rebalance of the serum free light chains after both HSCTs. The mechanism of HSCT-induced CIDP remission is not yet defined. Based on the normalization of the serum-free light chains kappa and lambda regarding absolute values and ratio after both HSCTs, we speculate that it may be attributed to the regression of the immune-mediated mechanisms related to the shrinking MGUS clone.
Our report confirms the positive impact of melphalan conditioning preceding autologous HSCT in patients with MGUS-associated neurologic disorders.
The favorable outcome of our approach at relapsing emphasizes to further explore adding lenalidomide for induction and post-transplant maintenance therapy in case of CIDP and concurrent IgG kappa MGUS. Our report may as well suggest to investigate other novel myeloma-specific therapeutic approaches in clinically refractory CIDP with concurrent MGUS, e.g., the anti-CD38 antibody daratumumab and venetoclax, a selective, orally bioavailable B-cell lymphoma 2 inhibitor that induces cell death in multiple myeloma cells, particularly in those harboring t(11;14), that express high levels of B-cell lymphoma 2 relative to B-cell lymphoma-extra large and myeloid leukemia cell 1.
Acknowledgment
Mrs. Daniela Rusca-Vitulano, Clinica Luganese Moncucco, assisted with figure preparation. Liliane Petrini, Ospedale Regionale di Lugano Civico e Italiano, performed technical editing and manuscript submission.
Appendix. Authors
Study funding
This study did not receive funding.
Disclosure
G. Colucci, T. Pabst, U. Bacher, C. Maggioli, and C. Zecca report no disclosures. The Department of Neurology, Regional Hospital Lugano (EOC), Lugano, Switzerland receives financial support from AbbVie, Almirall, Biogen Idec, Celgene, Lilly, Merck, Novartis, Sanofi, and Teva Pharma. C. Gobbi reports no disclosures. The Department of Neurology, Regional Hospital Lugano (EOC), Lugano, Switzerland, receives financial support from AbbVie, Almirall, Biogen Idec, Celgene, Lilly, Merck, Novartis, Sanofi, and Teva Pharma. Go to Neurology.org/NN for full disclosures.
References
- 1.Rajabally YA, Attarian S. Chronic inflammatory demyelinating polyneuropathy and malignancy: a systematic review. Muscle Nerve 2018;57:875–883. [DOI] [PubMed] [Google Scholar]
- 2.Alkhawajah NM, Dunnigan SK, Bril V. Comparison of monoclonal gammopathy of undetermined significance-associated neuropathy and chronic inflammatory demyelinating polyneuropathy patients. J Neurol 2014;261:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of paraproteinemic demyelinating neuropathies. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst 2010;15:185–195. [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC; Medscape. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol 2011;7:507–517. [DOI] [PubMed] [Google Scholar]
- 5.Puyade M, Labeyrie C, Badoglio M, et al. . Indication of autologous stem cell transplantation in chronic inflammatory demyelinating polyneuropathy: guidelines from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) [in French]. Bull Cancer 2020;107:S104–S113. [DOI] [PubMed] [Google Scholar]
- 6.Lee YC, Came N, Schwarer A, Day B. Autologous peripheral blood stem cell transplantation for peripheral neuropathy secondary to monoclonal gammopathy of unknown significance. Bone Marrow Transpl 2002;30:53–56. [DOI] [PubMed] [Google Scholar]
- 7.Van den Bergh PY, Hadden RD, Bouche P, et al. . European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. Eur J Neurol 2010;17:356–363. [DOI] [PubMed] [Google Scholar]