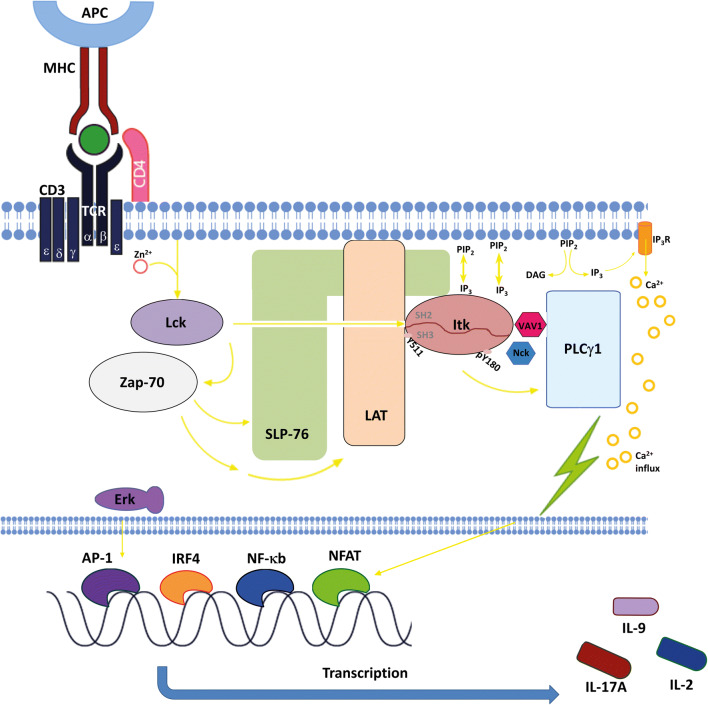
Fig. 1.
Interleukin-2-inducible T cell kinase plays a central role in the signal transduction of the T cell receptor. By binding of the MHC to the TCR, the kinase Lck (lymphocyte-specific protein tyrosine kinase) becomes activated with the help of Zn2+. Lck binds to protein Zap-70 (zeta-chain-associated protein kinase 70) which leads to the phosphorylation of LAT (linker for activation of T cells) and SLP-76 (SRC-homology-2-domain-containing leukocyte protein of 76 kDa). Upon binding of ITK to PIP3 (phosphatidylinositol-3,4,5-triphosphate) via its PH domain, ITK is recruited to the LAT/SLP-76-signalling complex. ITK interacts with SLP-76 and LAT at its SH2 and SH3 domains and gets phosphorylated on the tyrosine residue Y511 and Y180. The LAT/SLP-76 complex can also function as an accumulation platform for complex proteins like VAV1 (vav guanine nucleotide exchange factor 1) and Nck (non-catalytic region of tyrosine kinase). ITK becomes activated which results in the phosphorylation of PLCγ1 (phospholipase Cγ1) and the generation of IP3 (inositol-1,4,5-triphosphate) and DAG (diacylglycerol), which activates the PKC (protein kinase C). Finally, a Ca2+ influx within the cell can be observed. Through this signalling pathway, ITK can control the nuclear translocation of transcription factors AP-1 (activator protein 1) via Erk (extracellular-signal-regulated kinase), NFAT (nuclear factor of activated T cells), IRF4 (Interferon Regulatory Factor 4) and NF-κb (nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells) as well as the subsequent expression of various genes, e.g., IL-2, IL-9 and IL-17A