Abstract
Background:
Hepatocyte growth factor (HGF) has been shown to facilitate vocal fold (VF) wound healing. This study was undertaken to determine whether the therapeutic efficacy of HGF could be enhanced by applying it in hyaluronic acid and alginate (HA/ALG) composite hydrogels into VFs after injury in a rabbit model.
Methods:
HGF was loaded into HA/ALG composite hydrogel (HGF–HA/ALG) and its in vitro release profile was evaluated. In addition, HGF–HA/ALG was injected into the VFs of rabbits immediately after direct injury and HGF or PBS was injected in the same manner into control groups. Macroscopic features were observed by endoscopy at 3 months post-injury. Functional analyses including mucosal waves of VFs and viscoelastic properties were performed by kymography following high-speed digital imaging and rheometer. Histopathological and immunohistochemical evaluations were also conducted on VFs.
Results:
HGF release from HGF–HA/ALG was sustained for up to 3 weeks. Rabbits treated with HGF–HA/ALG showed improved mucosal vibrations and VF viscoelastic properties as compared with the PBS and HGF controls. Histopathological staining revealed HGF–HA/ALG treated VFs showed less fibrosis than PBS and HGF controls, and immunohistochemical analysis demonstrated amounts of type I collagen and fibronectin were lower in HGF–HA/ALG treated animals than in PBS and HGF controls at 3 months post-injury.
Conclusion:
HGF containing HA/ALG hydrogel enhanced healing in our rabbit model of VF injury.
Keywords: Hepatocyte growth factor, Hyaluronic acid, Alginate, Vocal fold, Wound healing
Introduction
Vocal folds (VFs) are connective tissues composed of a complex extracellular matrix (ECM). They have a unique layered structure, the biomechanics of which are determined by ECM components that importantly determine voice quality. VFs scars are the result of injuries caused by, for example, vocal abuse, surgery, or inflammation, and lead to VF vibration reduction and possibly dysphonia [1]. These voice disorders lead to changes in the tissues of the vocal cord mucosa. Excessive collagen deposition and reduced hyaluronan or elastin have been observed in the lamina propria [2]. VFs scars is one of the most challenging voice disorders and about 1.96% of the population in South Korea has abnormal laryngoscopic findings [3]. The remodeling of ECM composition by enhancing the functions of VF fibroblasts to produce ECM components has been focused on alleviating scarring and regenerating the VFs during the wound healing phase. Many studies based on principles of tissue engineering have been conducted to prevent and repair VF scar formation. These studies used cells, bioactive factors, and scaffolds to modulate the composition and distribution of ECM and redirect the fibrotic wound healing process [4]. Recently, various bioactive factors have been investigated for their ability to accelerate VF wound healing. The administration of growth factors in injured VF showed improved histological and biomechanical findings in animal models. Growth factors are believed to affect VF fibroblasts to promote ECM synthesis during the wound healing process [5–7]. However, the detail roles of the growth factor involved in the stage of VF wound healing have not been fully elucidated.
Hepatocyte growth factor (HGF) reduces fibrosis and has been suggested to be a relevant bioactive factor in the context of VF regeneration [8]. HGF influences VF fibroblasts by stimulating hyaluronic acid (HA) synthesis and reducing collagen production for a few weeks after injury, and thus, ameliorates VF scarring [9]. However, The therapeutic use of HGF within VF scarred tissues is limited by its short half lives (less than 5 min) and narrow therapeutic window [10], and thus, a new strategy is needed to ensure HGF levels in injured VFs are maintained for long enough to influence the organization of ECM molecules during scar formation. Thus, the sustained delivery of HGF coupled with the appropriate scaffold can be more effective on VFs repair and regeneration than the administration of HGF or scaffold alone. In fact, various naturally occurring and synthetic materials have been investigated with the aim of maximizing the effects of HGF [1, 11–13]. Na et al. previously investigated the potential of injectable hyaluronic acid and alginate (HA/ALG) composite hydrogels as carriers for sustained drug release [14]. In the present study, using a rabbit model, we investigated whether HGF released in vivo from HA/ALG composite hydrogels improves the functions of injured VFs after healing and whether these hydrogels are suitable HGF drug carriers.
Material and methods
Preparation of HA/ALG hydrogels containing HGF
HA solution (1 wt%) was prepared by dissolving HA in phosphate-buffered saline (PBS). ALG (1.2 wt%) and CaCl2 (0.13 wt%) were then added, respectively. HA-embedded ALG (HA/ALG) hydrogel was obtained as previously described [15]. HA chains are entrapped in the ALG network homogeneously. CaCl2 was used as a cross-linker for ALG. HA/ALG hydrogel containing 200 ng HGF/50 mL PBS was prepared for the in vivo experiment. All procedures were conducted under aseptic conditions.
In vitro release profile of HGF from HA/ALG hydrogels
HGF (PeproTech, Rocky Hill, NJ, USA)-loaded HA/ALG hydrogels (200 ul HA/ALG and 200 ng HGF) were pipetted into a 24-well culture plate containing 800 μL of phosphate buffer saline (PBS) (0.1 M, pH = 7.2 at 37 °C incubation). At indicated time points (1, 3, 7, 10, 14 and 21 days), the supernatant containing HGF was collected and the concentration of HGF was measured by an HGF-ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol.
Animal experiments
The study protocol was approved beforehand by the Animal Ethics Committee of Inha University Hospital. Animals were cared for in accordance with established institutional guidelines. Forty male New Zealand White rabbits with body weights ranging from 3.1 to 3.8 kg were used in the experiments. Animals were randomly divided into four groups: (1) an uninjured, non-treated group (the normal control group), (2) an injured and PBS-treated group (the PBS control group), (3) an injured and HGF treated group (the HGF group), or (4) an injured and HGF–HA/ALG-injected group (the HGF–HA/ALG group). For surgery, animals were subcutaneously premedicated with 0.05 mg/kg glycopyrrolate and 5 mg/kg xylazine and then anesthetized intramuscularly with 15 mg/kg zolazepam. A pediatric laryngoscope (Karl Storz, Tuttlingen, Germany) and an otomicroscope (Carl Zeiss Ltd., Welwyn Garden City, UK) were used to visualize larynges. Unilateral VF injury was conducted with a 2-mm micro cup forceps and microscissors as previously described [16]. The excision of the VF epithelium and lamina propria was made and PBS (50 µl), HA/ALG (50 µl), or HGF (500 ng)—HA/ALG (50 µl) were injected into sites of VF injury immediately after injury using a syringe equipped with a 25-gauge long needle under direct vision of a pediatric laryngoscope and a surgical operating microscope. Unilateral VF injury was produced in five VFs per group for obtaining high-speed camera images and evaluating histological changes. Contralateral VFs were used as controls. Bilateral VF injuries were made to five VFs per group and same materials were injected into both vocal folds for rheological evaluations [17]. All animals survived during the procedure and checked their status during 2 weeks (Fig. 1).
Fig. 1.
Experimental flow chart of in vivo study design
Macroscopic evaluations and high-speed camera imaging
Endoscopic evaluations were performed on all groups, and scar formations on VFs were assessed macroscopically at 3 months post-injury. Larynges were then excised for mucosal wave visualization. High-speed camera imaging of VF vibrations was performed as previously described [18]. Mucosal wave oscillations were compared by calculating the pixels of amplitudes of a point in at the upper border of VF mucosa in the mid-coronal glottal plane on kymographs [1]. MetaMorph software (Molecular Devices Corp., Sunnyvale, CA, USA) was used to transfer mucosal wave oscillations to kymographs.
Functional rheometric evaluation
Dissected VFs were stored in normal saline at 37 °C for 1 to 2 h before viscoelastic measurements were taken. Functional rheometric evaluations were performed as previously described [19]. Elastic modulus (G′) and viscous modulus (G″) were calculated, and all functional assessments were performed in a blinded fashion.
Histopathological and immunohistochemical examinations
Histological analysis of injured larynges was performed at 3 months post-injury. Specimens were embedded in paraffin blocks and sectioned at four μm using a microtome along the coronal axis of the larynx and then stained with hematoxylin and eosin (H&E) and Masson’s trichrome (for collagen) using standard pathology department protocols. Immunohistochemical examinations were also conducted using primary antibodies against collagen type I (1:100 dilution; Abbiotec, San Diego, CA, USA), fibronectin (1:50 dilution; Abcam, Cambridge, UK), secondary Alexa fluor 488-conjugated anti-rabbit immunoglobulin G (IgG) and secondary Alexa fluor 488-conjugated anti-mouse IgG antibodies (dilutions 1:500; Invitrogen, Carlsbad, CA, USA). Sections were then stained with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen) for 3–5 min to stain cell nuclei. Slides were observed under a confocal laser-scanning microscope.
Statistics
All analyses were conducted using the GraphPad Prism 5 package (GraphPad Software Inc., La Jolla, CA, USA). Kruskal–Wallis test with Dunns’ post hoc test was used for group comparisons, and a two-way analysis of variance with Bonferroni’s post hoc test was used to analyze rheological data. Statistical significance was accepted for p values of < 0.05.
Results
Sustained release of HGF from HGF-loaded HA/ALG hydrogels
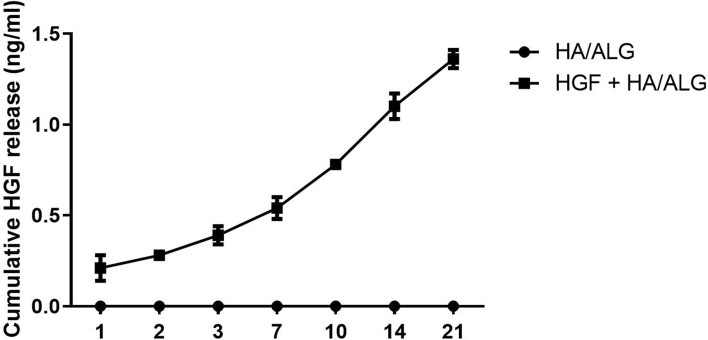
The release of HGF from HGF–HA/ALG over 21 days was evaluated. The cumulative amount of HGF increased consistently over this period showing release occurred in a sustained manner (Fig. 2).
Fig. 2.
Profile of hepatocyte growth factor (HGF) release from HA/ALG hydrogel. The amount of HGF released increased consistently over 21 days
Macroscopic evaluation
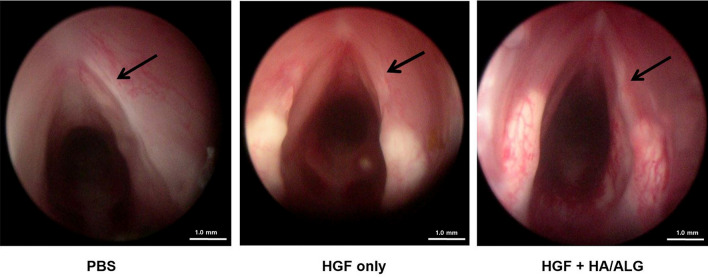
Endoscopic examination of VFs at 3 months post-injury revealed prominent fibrotic scars in the PBS group, but less scar formation in the HGF and HGF–HA/ALG groups. No implant-or procedure-related complication was observed (Fig. 3).
Fig. 3.
Endoscopic examination of VFs at 3 months post-injury. Endoscopic examination of VFs at 3 months post-injury revealed prominent fibrotic scars in the PBS group, but reduced scar formation in the HGF and HGF–HA/ALG groups (the PBS group; injured and PBS treated, the HGF group; injured and HGF treated, HGF–HA/ALG group; injured and HGF–HA/ALG treated)
Micromorphological evaluation
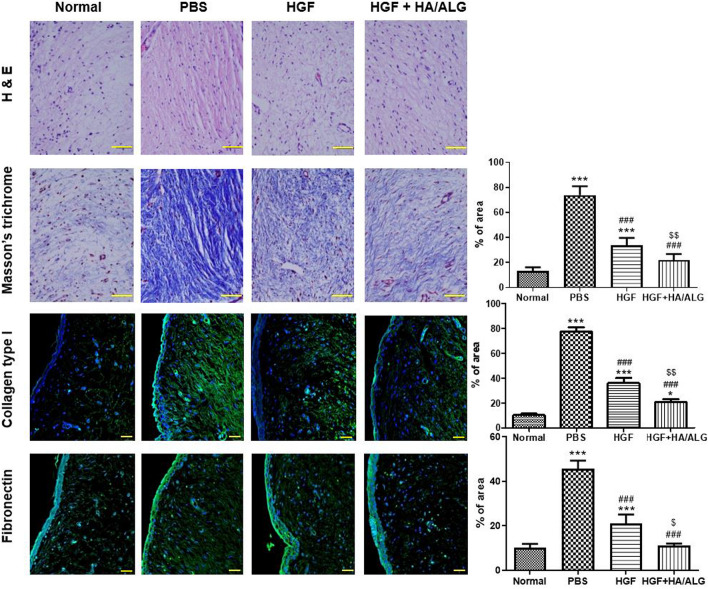
Changes in ECM were assessed 3 months post-injury (Fig. 4). H&E and Masson’s trichrome staining showed that HGF had favorable effects on injured VFs. Masson’s trichrome staining revealed considerably more fibrosis in the PBS group than in normal controls, but obviously less fibrosis in HGF and HGF–HA/ALG groups than in the PBS group. In addition, collagen levels were lower in the HGF–HA/ALG group than in the HGF group. Immunohistochemical analysis of ECM components showed more significant collagen type I and fibronectin depositions in the PBS group than in normal controls (Fig. 4), less collagen type I and fibronectin filing the HGF group than in the PBS group, and less collagen type I and fibronectin deposition the HGF–HA/ALG group than in the HGF group.
Fig. 4.
Micromorphological and immunohistochemical evaluations of ECM remodeling by HGF released from HGF–HA/ALG at 3 months post-injury. Scale bars represent 30 μm. Masson's trichrome (blue-stained collagen), collagen type I and fibronectin densities were quantified by summing numbers of pixels in positively stained areas using a software program. Results are presented as mean areas (%) ± SDs as determined by the Kruskal–Wallis test with Dunns’ post hoc test. *versus normal uninjured/non-treated controls, #versus injured/PBS treated animals, $versus injured/HGF treated animals, *p < 0.05, ***p < 0.001, ###p < 0.001, $p < 0.05 and $$p < 0.01. (n = 5 rabbits per group) (the normal control group; non-injured and non-treated, the PBS group; injured and PBS treated, the HGF group; injured and HGF treated, HGF–HA/ALG group; injured and HGF–HA/ALG treated)
Evaluation of kymographs and mucosal waves
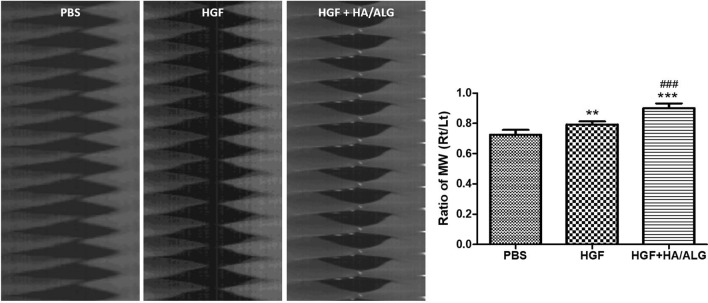
Mucosal waves of excised larynges were recorded using a high-speed digital camera (Fig. 5). The ratio of VF oscillation of treated to control VFs in the PBS group decreased 3 months post-injury. Mucosal waves were improved in the HGF and HGF–HA/ALG groups as compared with the PBS group, and the mucosal oscillation ratio in the HGF–HA/ALG group was significantly higher than in the PBS group. Furthermore, mucosal waves were much improved in the HGF–HA/ALG group as compared with the HGF group.
Fig. 5.
Evaluation of mucosal wave oscillations after vocal fold injury. The amplitudes of mucosal waves (MWs) of VFs were evaluated by measuring the pixels of amplitudes of a mid-point of upper lips on high-speed digital images using image analysis software at 3 months post-injury. The amplitude ratios of MWs of treated VFs versus normal controls were calculated using Metamorph software. Normalized MW results are presented as mean ratios (right VFs to left VFs) ± SDs. Kruskal–Wallis test with Dunns’ post hoc test. *versus the PBS group, #versus the HGF group, **p < 0.01, ***p < 0.001 and ###p < 0.001 (n = 5 rabbits per group) (PBS group; injured and PBS treated, HGF group; injured and HGF treated, HGF–HA/ALG group; injured and HGF–HA/ALG treated)
Evaluation of the biomechanical properties of VFs
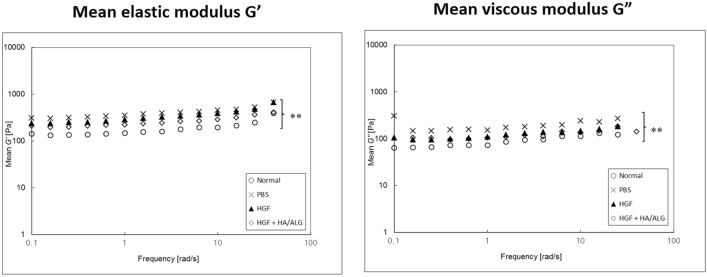
Functional viscoelastic properties [elastic moduli (G′) and viscous moduli (G″)] were calculated and plotted on a log–log scale as a function of frequency for all groups (Fig. 6). The PBS group had significantly higher G′ and G″ values than normal controls and the HGF and HGF–HA/ALG groups had significantly lower G′ and G″ values than the PBS group and the HGF–HA/ALG group lower values than the HGF group. Relationships between viscoelastic properties and frequency modeled using G′ or G″ = aXb, where ‘a’ was the magnitude and ‘b’ was a slope on a log–log scale. Curve fitting results are shown in Table 1.
Fig. 6.
Rheometric evaluations of viscoelasticity changes after vocal fold injury. Viscoelastic properties of regenerated VFs were measured at 3 months post-injury. Mean elastic moduli (G′) and viscous moduli (G″) are plotted on a log–log scale as a function of frequency. Rheological data were analyzed at all frequencies by two-way ANOVA with Bonferroni’s post hoc test (both p < 0.01) (n = 5 rabbits per group) (the normal control group; non-injured and non-treated, the PBS group; injured and PBS treated, the HGF group; injured and HGF treated, HGF–HA/ALG group; injured and HGF–HA/ALG treated)
Table 1.
Results of curve fitting using the power law of plots between mean elastic modulus (G′) or mean viscous modulus (G″) and applied frequency in the four study groups
| a (Pa s) | b | R2 | |
|---|---|---|---|
| G′ = aXb | |||
| Normal | 173 ± 72 | 0.098 | 0.647 |
| PBS | 332 ± 89 | 0.114 | 0.871 |
| HGF | 283 ± 53 | 0.138 | 0.866 |
| HGF + HA/ALG | 233 ± 156 | 0.133 | 0.843 |
| G″ = aXb | |||
| Normal | 63 ± 27 | 0.143 | 0.487 |
| PBS | 175 ± 55 | 0.121 | 0.877 |
| HGF | 102 ± 32 | 0.08 | 0.507 |
| HGF + HA/ALG | 103 ± 25 | 0.195 | 0.851 |
The normal control group; non-injured and non-treated, the PBS group; injured and PBS treated, the HGF group; injured and HGF treated, HGF–HA/ALG group; injured and HGF–HA/ALG treated
Discussions
Many studies have been carried out to minimize VF fibrosis and restore ECM components and biomechanics in injured VFs using bioactive molecules or scaffolds. In the present study, we investigated whether HGF loaded HA/ALG hydrogels (HGF–HA/ALG) could promote VF wound healing and improve VF functions after injury. Recently, injectable hydrogel systems have attracted research attention as potential drug delivery vehicles [20–22]. The gel systems investigated have contained various therapeutic agents including bioactive factors and growth factors. HA is widely used as a delivery carrier and is a major ECM component and abundant in normal VFs. HA plays critical regulatory roles in cell growth and renewal and has been shown to accelerate wound healing [23]. In addition, it has been reported to promote fibroblastic differentiation and to be effective at improving the VF function [24]. On the other hand, ALG is a natural polymer that is widely used as a carrier for the delivery of growth factors and has excellent biocompatibility and is relatively cheap [25, 26]. In the present study, the HA/ALG composite hydrogel was easily prepared for injection by simply mixing it with HGF. Our in vitro results showed HA/ALG hydrogel facilitated HGF retention and viability by providing sustained HGF release for up to 3 weeks.
HGF plays an essential role in wound healing by enhancing cellular proliferation, migration, and angiogenesis [27]. It has been demonstrated to be a useful anti-fibrotic agent in scarred VFs [1, 12], and shown to stimulate HA production and inhibit disorganized collagen deposition in scarred VFs, and thereby, facilitate VF regeneration [9, 28]. However, HGF has a narrow therapeutic window and short half-life in vivo, and thus, we developed a biodegradable HA/ALG hydrogel that provides sustained HGF release and tested its ability to promote wound repair in a rabbit model of VF injury. The resulting HGF loaded hydrogel provided a sustained release for up to 21 days in vitro. We believe that the loaded HGF will have a positive effect on the fibroblasts in the vocal cords continuously during fibroblast growth proliferation phase (about 21 days) [29]. However, further study is required to know exact concentration of HGF for regeneration of vocal folds. Also, confirming the bioactivity of released HGF from HA/ALG hydrogel will be necessary as a further research.
Excessive collagen and fibronectin deposition adversely affect VF viscoelasticity. In VFs, type I collagen provides tensile strength and contributes to stiffness. Fibronectin is also an essential component of ECM and influences VF viscosity, and the presence of excessive fibronectin in lamina propria of scarred VFs probably importantly determines scarred VF stiffness because of its biological associations with binding, adhesion, and strength [30]. In the present study, we observed HGF–HA/ALG attenuated fibrosis, improved collagen alignment, and reduced fibronectin expression versus the PBS group in scarred VFs. Furthermore, HGF–HA/ALG reduced the expressions of collagen and fibronectin more than HGF. These observations suggest HGF–HA/ALG enhances VF healing by controlling ECM components and improving biomechanics, and that the lower levels of type I collagen and fibronectin observed in the HGF–HA/ALG group increased the tissue viscoelasticity and pliability of VF mucosa.
We also found that HGF–HA/ALG better restored the functional biomechanical properties of injured VFs than PBS or HGF alone. Functional improvements in viscoelastic properties and mucosal wave oscillations elicited by HGF–HA/ALG appeared to be associated with ECM redistribution in injured VFs. Rheological characteristics in the HA/ALG group were similar to those of the normal control group, showing HA/ALG hydrogel had little effect on stiffness or resistance to mucosal flow as previous study [31]. Caton et al. suggested biomaterials with viscoelastic properties similar to human VF mucosa appear to be appropriate for superficial injection into the VFs [32]. In this regard, HA/ALG hydrogel seems to be a suitable injection material for VFs. However, HA/ALG hydrogel injected group was not included and it is a limitation in our study.
In the present study, we developed a biodegradable HA/ALG hydrogel that retained and sustainably released HGF during VF wound healing. HGF–HA/ALG treatment was found to consistently provide greater functional VF remodeling post-injury than HGF alone. Our results suggest that the devised injectable HA/ALG hydrogel acts as a useful drug carrier that overcomes the limitations of HGF alone for the treatment of VF repair.
Acknowledgement
This research was supported by the Medical Research Center (MRC) (NRF-2014R1A5A2009392) of the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP).
Compliance with ethical standards
Conflict of interest
The authors have no potential conflict of interest to declare.
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Inha University. (IACUC approval No. INHA 141003-330-3).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi JS, Lee S, Kim DY, Kim YM, Kim MS, Lim JY. Functional remodeling after vocal fold injury by small intestinal submucosa gel containing hepatocyte growth factor. Biomaterials. 2015;40:98–106. doi: 10.1016/j.biomaterials.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20:110–120. doi: 10.1016/j.jvoice.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Woo SH, Kim RB, Choi SH, Lee SW, Won SJ. Prevalence of laryngeal disease in South Korea: data from the Korea National Health and Nutrition Examination Survey from 2008 to 2011. Yonsei Med J. 2014;55:499–507. doi: 10.3349/ymj.2014.55.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long JL. Tissue engineering for treatment of vocal fold scar. Curr Opin Otolaryngol Head Neck Surg. 2010;18:521–525. doi: 10.1097/MOO.0b013e32833febf2. [DOI] [PubMed] [Google Scholar]
- 5.Hirano S, Bless DM, Rousseau B, Welham N, Montequin D, Chan RW, et al. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004;114:548–556. doi: 10.1097/00005537-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Suehiro A, Hirano S, Kishimoto Y, Rousseau B, Nakamura T, Ito J. Treatment of acute vocal fold scar with local injection of basic fibroblast growth factor: a canine study. Acta Otolaryngol. 2010;130:844–850. doi: 10.3109/00016480903426618. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, Kobler JB, Zeitels SM, Langer R. Effects of growth factors on extracellular matrix production by vocal fold fibroblasts in 3-dimensional culture. Tissue Eng. 2006;12:3365–3374. doi: 10.1089/ten.2006.12.3365. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto Y, Hirano S, Suehiro A, Tateya I, Kanemaru S, Nakamura T, et al. Effect of exogenous hepatocyte growth factor on vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2009;118:606–611. doi: 10.1177/000348940911800813. [DOI] [PubMed] [Google Scholar]
- 9.Hirano S, Bless D, Heisey D, Ford C. Roles of hepatocyte growth factor and transforming growth factor beta1 in production of extracellular matrix by canine vocal fold fibroblasts. Laryngoscope. 2003;113:144–148. doi: 10.1097/00005537-200301000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Moreno E, Meneu JC, Calvo J, Pérez B, Sesma AG, Manrique A, et al. Modulation of hepatocyte growth factor plasma levels in relation to the dose of exogenous heparin administered: an experimental study in rats. Transplant Proc. 2005;37:3943–3947. doi: 10.1016/j.transproceed.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 11.Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS. Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction. J Biomed Mater Res A. 2010;93:1335–1347. doi: 10.1002/jbm.a.32632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JW, Kim YS, Park JK, Song EH, Park JH, Kim MS, et al. Controlled release of hepatocyte growth factor from MPEG-b-(PCL-ran-PLLA) diblock copolymer for improved vocal fold regeneration. Macromol Biosci. 2017;17:1600163. doi: 10.1002/mabi.201600163. [DOI] [PubMed] [Google Scholar]
- 13.Kolachala VL, Henriquez OA, Shams S, Golub JS, Kim YT, Laroui H, et al. Slow-release nanoparticle-encapsulated delivery system for laryngeal injection. Laryngoscope. 2010;120:988–994. doi: 10.1002/lary.20856. [DOI] [PubMed] [Google Scholar]
- 14.Na SY, Oh SH, Song KS, Lee JH. Hyaluronic acid/mildly crosslinked alginate hydrogel as an injectable tissue adhesion barrier. J Mater Sci Mater Med. 2012;23:2303–2313. doi: 10.1007/s10856-012-4689-0. [DOI] [PubMed] [Google Scholar]
- 15.Oh SH, Kim JK, Song KS, Noh SM, Ghil SH, Yuk SH, et al. Prevention of postsurgical tissue adhesion by anti-inflammatory drug-loaded pluronic mixtures with sol-gel transition behavior. J Biomed Mater Res A. 2005;72:306–316. doi: 10.1002/jbm.a.30239. [DOI] [PubMed] [Google Scholar]
- 16.Kim YM, Yi T, Choi JS, Lee S, Jang YH, Kim CH, et al. Bone marrow-derived clonal mesenchymal stem cells as a source of cell therapy for promoting vocal fold wound healing. Ann Otol Rhinol Laryngol. 2013;122:121–130. doi: 10.1177/000348941312200208. [DOI] [PubMed] [Google Scholar]
- 17.Kanemaru S, Nakamura T, Yamashita M, Magrufov A, Kita T, Tamaki H, et al. Destiny of autologous bone marrow-derived stromal cells implanted in the vocal fold. Ann Otol Rhinol Laryngol. 2005;114:907–912. doi: 10.1177/000348940511401203. [DOI] [PubMed] [Google Scholar]
- 18.Lim JY, Choi BH, Lee S, Jang YH, Choi JS, Kim YM. Regulation of wound healing by granulocyte-macrophage colony-stimulating factor after vocal fold injury. PLoS One. 2013;8:e54256. doi: 10.1371/journal.pone.0054256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi JS, Kim NJ, Klemuk S, Jang YH, Park IS, Ahn KH, et al. Preservation of viscoelastic properties of rabbit vocal folds after implantation of hyaluronic acid-based biomaterials. Otolaryngol Head Neck Surg. 2012;147:515–521. doi: 10.1177/0194599812446913. [DOI] [PubMed] [Google Scholar]
- 20.Chaterji S, Kwon IK, Park K. Smart polymeric gels: redefining the limits of biomedical devices. Prog Polym Sci. 2007;32:1083–1122. doi: 10.1016/j.progpolymsci.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung SW, Oh SH, Lee IS, Byun JH, Lee JH. In situ gelling hydrogel with anti-bacterial activity and bone healing property for treatment of osteomyelitis. Tissue Eng Regen Med. 2019;16:479–90. doi: 10.1007/s13770-019-00206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahapatra C, Jin GZ, Kim HW. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng Regen Med. 2016;13:538–46. doi: 10.1007/s13770-016-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H, Karajanagi S, Wolak K, Aanestad J, Daheron L, Kobler JB, et al. Three-dimensional hydrogel model using adipose-derived stem cells for vocal fold augmentation. Tissue Eng Part A. 2010;16:535–543. doi: 10.1089/ten.tea.2009.0029. [DOI] [PubMed] [Google Scholar]
- 25.Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J Control Release. 2009;134:26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohta M, Suzuki Y, Chou H, Ishikawa N, Suzuki S, Tanihara M, et al. Novel heparin/alginate gel combined with basic fibroblast growth factor promotes nerve regeneration in rat sciatic nerve. J Biomed Mater Res A. 2004;71:661–668. doi: 10.1002/jbm.a.30194. [DOI] [PubMed] [Google Scholar]
- 27.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 29.Garg A, Yuen S, Seekhao N, Yu G, Karwowski JAC, Powell M, et al. Towards a physiological scale of vocal fold agent-based models of surgical injury and repair: sensitivity analysis, calibration and verification. Appl Sci (Basel) 2019;9:2974. doi: 10.3390/app9152974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirano S, Bless DM, Rousseau B, Welham N, Scheidt T, Ford CN. Fibronectin and adhesion molecules on canine scarred vocal folds. Laryngoscope. 2003;113:966–972. doi: 10.1097/00005537-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Kim YM, Oh SH, Choi JS, Lee S, Ra JC, Lee JH, et al. Adipose-derived stem cell-containing hyaluronic acid/alginate hydrogel improves vocal fold wound healing. Laryngoscope. 2014;124:E64–72. doi: 10.1002/lary.24405. [DOI] [PubMed] [Google Scholar]
- 32.Caton T, Thibeault SL, Klemuk S, Smith ME. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117:516–521. doi: 10.1097/MLG.0b013e31802e9291. [DOI] [PubMed] [Google Scholar]