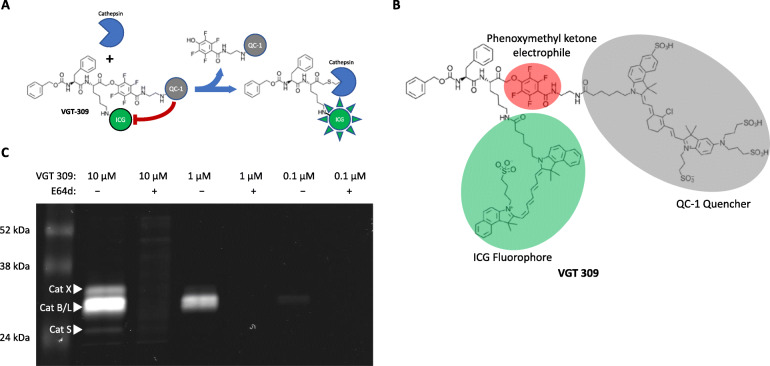
Fig. 1.
Mode of action and structure of VGT-309 and in vitro binding to active cysteine cathepsins. a Mode of action of VGT-309. Cathepsins covalently and irreversibly bind VGT-309, displacing the quencher QC-1. With the quencher displaced, the ICG fluorescence of VGT-309 is no longer quenched and the probe becomes fluorescent. b Structure of VGT-309. The phenoxymethyl ketone electrophile (red) forms a covalent bond with the active cysteine of cathepsins. The fluorophore ICG (green) is for visualization and the quencher (gray) prevents fluorescent signal in the free, unbound probe. c VGT-309 labeling of endogenously expressed cathepsins in RAW-264.7 cells. Cells were treated with probe for 30 min in the presence or absence of cysteine cathepsin inhibitor E64d followed by lysis and analysis by SDS-PAGE and scanning for fluorescent labeled proteins. The locations of cathepsin X, B/L, and S are indicated