Figure 4. Strategies to introduce reactive functional groups in glycosphingolipids.
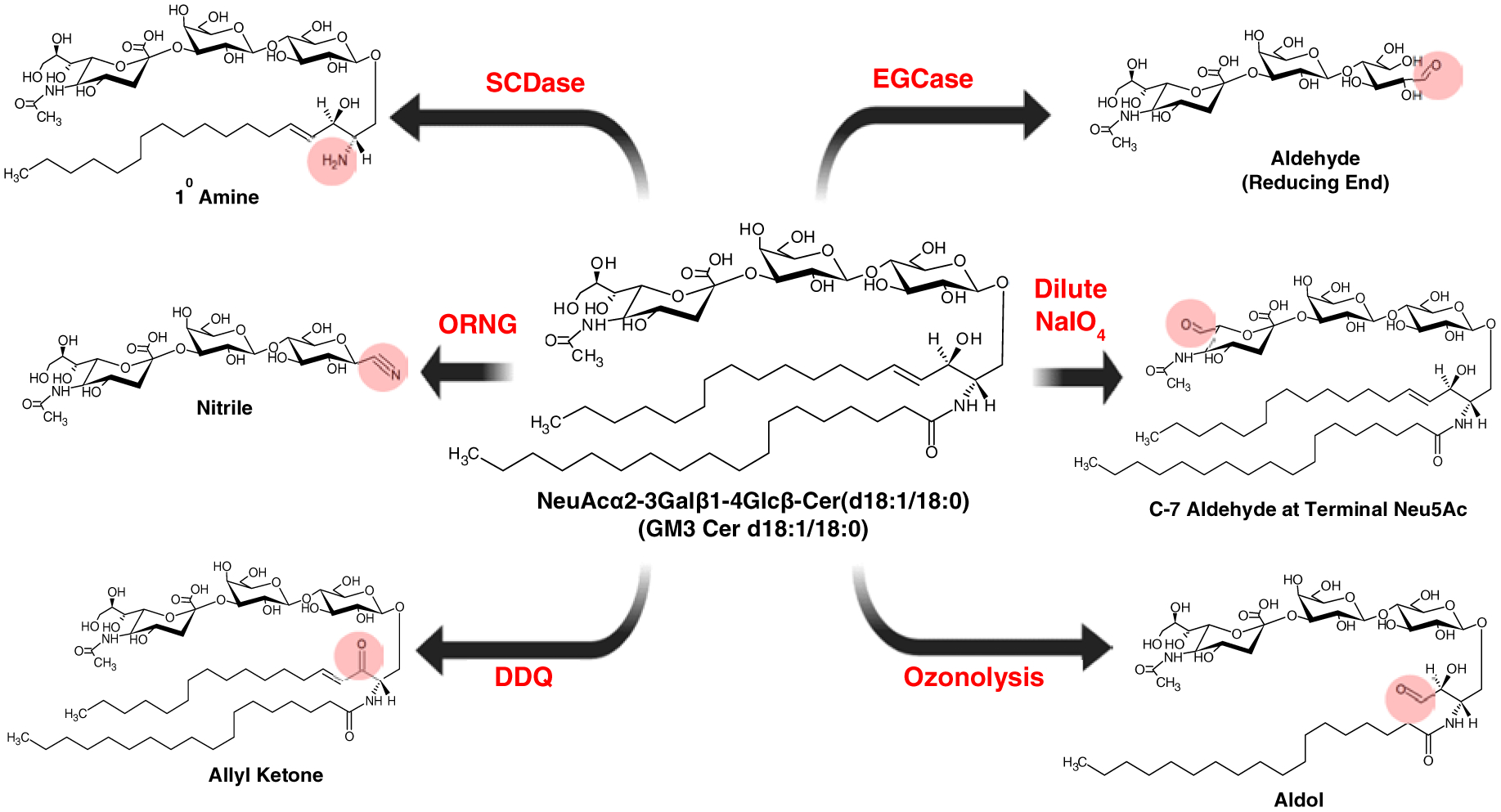
Sphingoid N-deacylase (SCDase) is used to remove the fatty acyl chain leaving behind the sphingosine backbone. Endoglycoceraminidase (EGcase) is used to remove the lipid part leaving the reducing end of the glycan intact. Oxidative release of natural glycans (ORNG) uses bleach (NaOCl) to convert the rest of the lipid tail to a nitrile group, which could be further reduced to form a primary amine group and used for other chemistries. Sodium meta periodate (NaIO4) can selectively oxidize the trihydroxyfunctional group of sialic acid to aldehyde. Selective oxidation of the sphingosine double bond can be accomplished using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). Ozonolysis cleaves the C=C on the sphingosine but could also cleave other C=C bonds if present.
