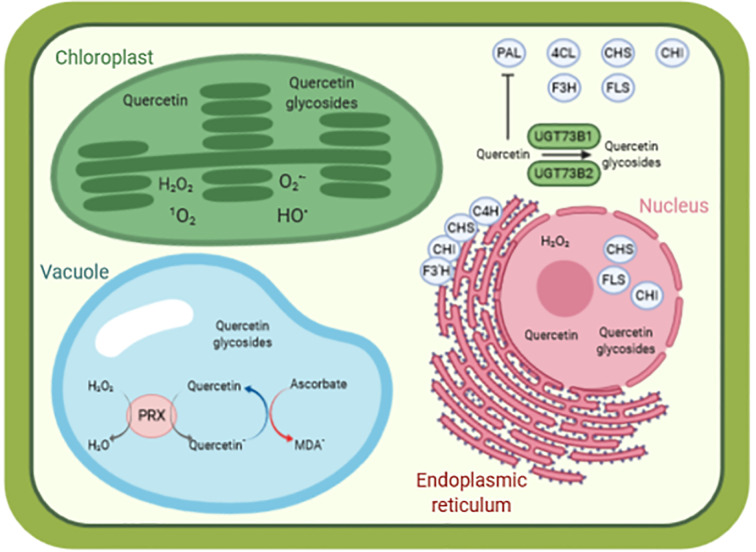
Figure 2.
A simplified model of quercetin as antioxidant molecule in a cellular context. The biosynthesis of quercetin is performed by enzymes located in the cytosol (PAL, C4H, CHS, F3H, FLS), anchored to the endoplasmic reticulum (C4H, CHS, CHI, F3’H) and present in the nucleus (CHS, CHI, FLS). Quercetin is glycosylated by various UGTs, such as UGT73B1 and UGT73B2 in Arabidopsis thaliana. Quercetin and quercetin glycosides are found in various compartments (such as cytosol, nucleus, chloroplasts, and vacuole), contributing to redox homeostasis and preventing oxidative damages caused by reactive species, for instance, in the chloroplasts. In the vacuole, quercetin reduces H2O2 to H2O in a peroxidase-dependent reaction, leading to the formation of quercetin radical. This radical is then recycled back to quercetin by oxidation of ascorbate to monodehydroascorbate radical. Data from (Burbulis and Winkel-Shirley, 1999; Saslowsky et al., 2005; Fujino et al., 2018; Khorobrykh et al., 2020). 1O2 singlet state of molecular oxygen, HO• hydroxyl radical, H2O2 hydrogen peroxide, and O2•− superoxide anion radical. 4CL, 4-coumaroyl:CoA-ligase; C4H, cinnamate 4-hydroxylase; CHI, chalcone isomerase; CHS, chalcone synthase; F3H, flavanone 3-hydroxylase; F3’H, flavonoid 3’-hydroxylase; FLS, flavonol synthase; MDA•, monodehydroascorbate radical; PAL, phenylalanine ammonia lyase; PRX, peroxidase. Picture designed with Biorender.