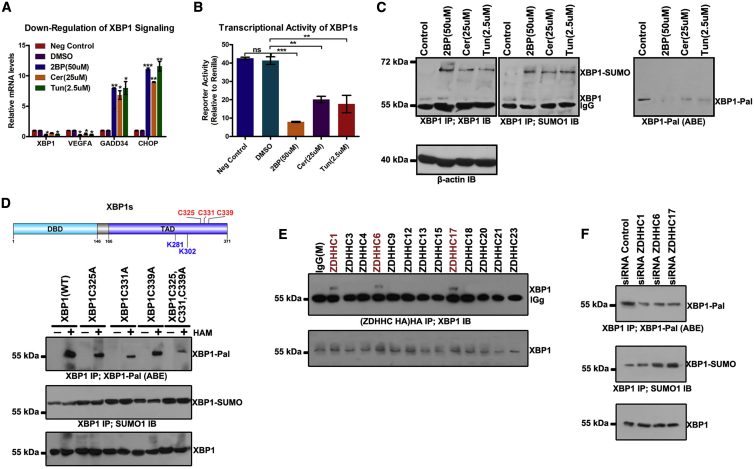
Figure 7.
X-box Binding Protein 1 (XBP1) Signaling Is Downregulated by Palmitoylation Inhibitors in SF126 Cells
(A) SF126 cells were treated with 2BP (50 μM), Cer (25 μM), or Tun (2.5 μM) for 24 h. RNA was extracted from each sample and real-time polymerase chain reaction was performed to analyze the levels of spliced X-box binding protein 1 (XBP1s), vascular endothelial growth factor A (VEGFA), GADD34, and CCAAT-enhancer binding protein homologous protein (CHOP) mRNAs. (B) Palmitoylation inhibitors decreased the transcriptional activity of XBP1s. A 5× unfolded pathway response element-luciferase reporter and XBP1s expression construct were used to determine the transcriptional activity of XBP1s. The firefly luciferase value was divided by the Renilla luciferase value to normalize each sample. Data are expressed as means ± SD (n = 3). (C) Increased accumulation of SUMOylated XBP1 in 2BP (50 μM), Cer (25 μM), or Tun (2.5 μM) treatment versus that in control group. XBP1 was immunoprecipitated with anti-XBP1 antibody (IP) from these cell lysates. Bound proteins were blotted with anti-XBP1 or anti-SUMO1 antibody (IB). (D) Cys325, Cys331, and Cys339 were determined as XBP1 palmitoylation sites. Palmitoylation sites Cys325, Cys331, and Cys339 of XBP1 were predicted using CSS-Palm 4.0 and mutated to Ala, respectively, the palmitoylation level of XBP1 was detected via the ABE method, and the SUMOylated XBP1 was also analyzed. (E) SF126 was transfected with hemagglutinin (HA)-tagged DHHC members upregulated in GBM, and subjected to immunoprecipitation (IP) of HA. (F) Palmitoylation and SUMOylation levels of XBP1 in SF126 cells transfected with siRNAs for different DHHCs. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, unpaired t test. ns, not significant.