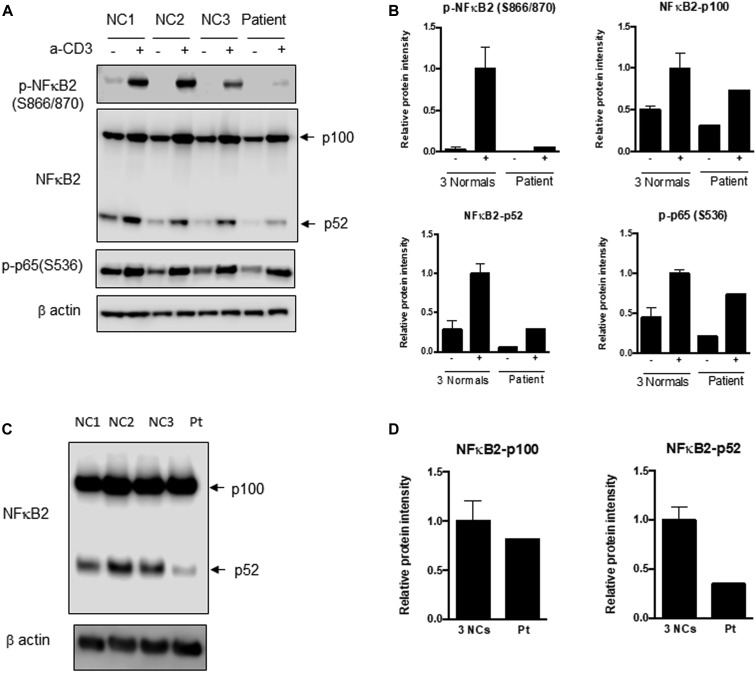
Fig 2.
Immunoblotting for NF-κB2, p52, and phosphorylated p65 (RelA). A, PBMCs from the patient (Pt) and healthy controls 1 to 3 (NC 1, NC 2, and NC3) were stimulated with soluble anti-CD3. Immunoblotting was performed for NF-κB2 precursor (p100) and the processed form (p52), phosphorylated NF-κB2, and phosphorylated p65 (RelA) of the canonical pathway in both stimulated and unstimulated samples. There is a distinct inability to phosphorylate NF-κB2, and the amount of processed p52 protein is substantially reduced in the patient sample following stimulation. B, Densitometric analysis of the immunoblot in (A). C, T-cell blasts were generated from PBMCs by stimulation with anti-CD3 and anti-CD28 with IL-2. Blast cell lysates were analyzed for p100 and p52 of NF-κB2. D, Bar graphs depict the relative expression levels of these proteins normalized to β-actin by densitometry. Compared with in normal controls, there is a small decrease in p100 but a remarkable reduction in the processed form, p52 protein, which correlates with the PBMC data. The extent of decrease in phospho-NF-κB2 and p52 is supportive of a complete deficiency for this variant.