Table 1.
Profile of antivirals investigated for COVID-19 treatment in clinical trials or in vitro studies. Source: WHO, adapted from landscape analysis 17 February 2020 https://www.who.int/blueprint/priority-diseases/key
| Group | Drugs | Structure and PubChem CID | Route of administration | Current use | Mechanism of action | EC50 value in Vero E6 cell | Being tested? |
|---|---|---|---|---|---|---|---|
| Inhibitors of viral RNA polymerase/RNA synthesis | Remdesivir | PubChem CID:121304016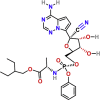
|
Intravenous | COVID-19 | A prodrug, adenosine nucleotide analogue, which acts as an RdRp inhibitor | 1.76 μM | Approved |
| Favipiravir | PubChem CID:492405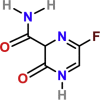
|
Intravenous | influenza | A prodrug, guanosine nucleotide analogue, which acts as an RdRp inhibitor | 61.88 μM | Yes | |
| Inhibitors of viral protein synthesis | Lopinavir/ritonavir | PubChem CID:11979606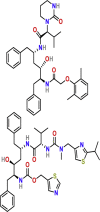
|
Orally | HIV | Inhibition of protease | 26.62 μM | Yes |
| Viral entry inhibitors | Hydroxychloroquine | PubChem CID:3652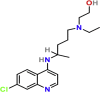
|
Orally | Antimalarial | Increase the endosomal pH required by the virus/Cell fusion, and interference Glycosylation of SARS-CoV (ACE-2) cell receptor | 4.51 μM | yes |
| Chloroquine | PubChem CID:2719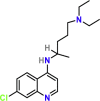
|
Orally | Antimalarial | 2.71 μM | |||
| Immunomodulatory | Nitazoxanide | PubChem CID:41684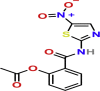
|
Orally | Anti-parasitic and antiviral | Interference involves viral replication, amplification of cytoplasmic RNA sensing, and host regulation pathways of type I interferon | 2.12 μM | yes |
| Ivermectin | PubChem CID:6321424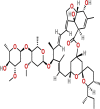
|
Orally | Anti-parasitic | Inhibit the import of nuclear proteins into hosts and viruses by suppressing importin 1 Heterodimer | NA | yes | |
| Immunomodulatory | Tocilizumab | NA | Intravenously | Rheumatoid arthritis; COVID-19 | inhibit signal transduction by specifically binding to sIL-6R and mIL-6R | NA | Approved |
| Anti-inflammatory | Dexamethasone | 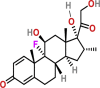 |
Intravenously and orally | Anti-inflammatory | Act as an inflammatory agent | NA | yes |
Note: Not applicable- NA.
