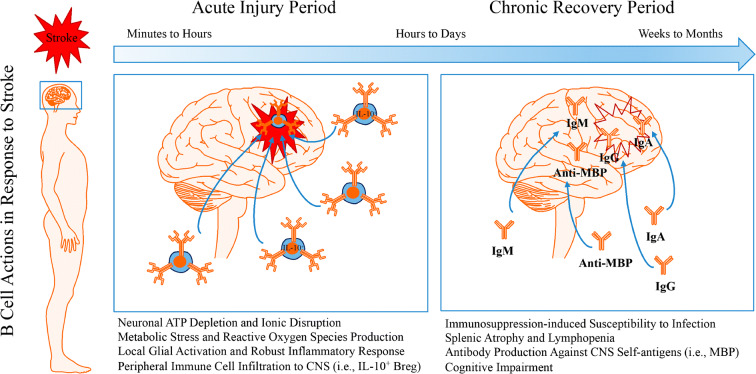
Fig. 2.
Dual roles for B cells in the acute and chronic periods following stroke. The pathological sequelae associated with stroke-induced restriction in blood flow and the post-occlusion reperfusion process induces neuronal metabolic dysfunction leading to an energy crisis in infarcted brain tissue. Depletion of ATP reserves, disruption to ion homeostasis, accumulation of cell-damaging reactive oxygen species, and excitotoxic states in brain parenchymal cell serve as danger/damage signals to induce an activated, proinflammatory phenotype in resident microglia. This proinflammatory state recruits peripheral immune cells, including B cells, to injured CNS tissues through leaky blood-brain barrier openings. During the acute phase, the activation of glial cells and the recruitment of peripheral leukocytes into the CNS exacerbate stroke pathology and worsen stroke outcome. Yet, B cell recruitment does not seem to contribute to stroke neuropathology and may actually serve protective roles during the acute injury period via interleukin (IL)-10-dependent immunoregulation. During the chronic recovery phase, CNS-resident and peripheral-originating CNS-infiltrated immune cells promote tissue repair and improve stroke outcome. However, B cell production of auto-reactive antibodies against various CNS antigens may contribute to the development of delayed post-stroke cognitive impairment. While immunomodulation represents a viable novel therapeutic approach for the treatment of stroke, additional studies are required to better understand the function of lymphocytes following ischemic stroke