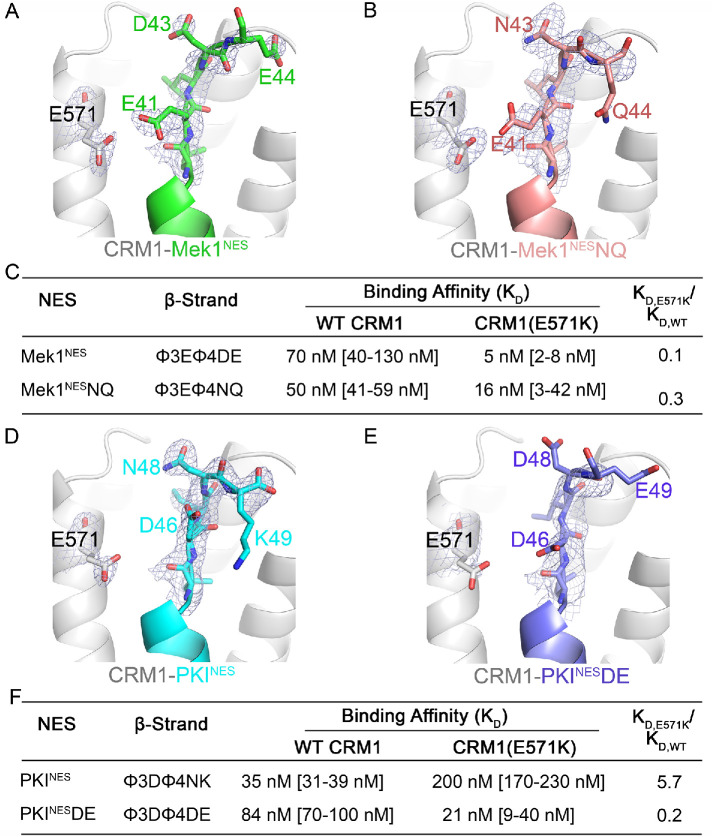
FIGURE 5:
Additional NES β-strand residues contribute interactions with WT and E571K CRM1. (A) Details of the NES-binding grooves of Mek1NES (green, left) and (B) Mek1NQNES (pink) bound to WT CRM1 (gray). (C) Binding affinities of interactions between wild type and mutated Mek1NES with WT and E571K CRM1. (D, E) Details of the NES-binding grooves of PKINES (cyan, D) and PKINESDE (pink, E) bound to WT CRM1 (gray). (F) Binding affinities of interactions between wild type PKINES and PKINESDE with WT and E571K CRM1. Electron density from composite omit maps (2Fo-Fc contoured in blue mesh to 1σ) are shown for β-strands in A, B, D, and E.