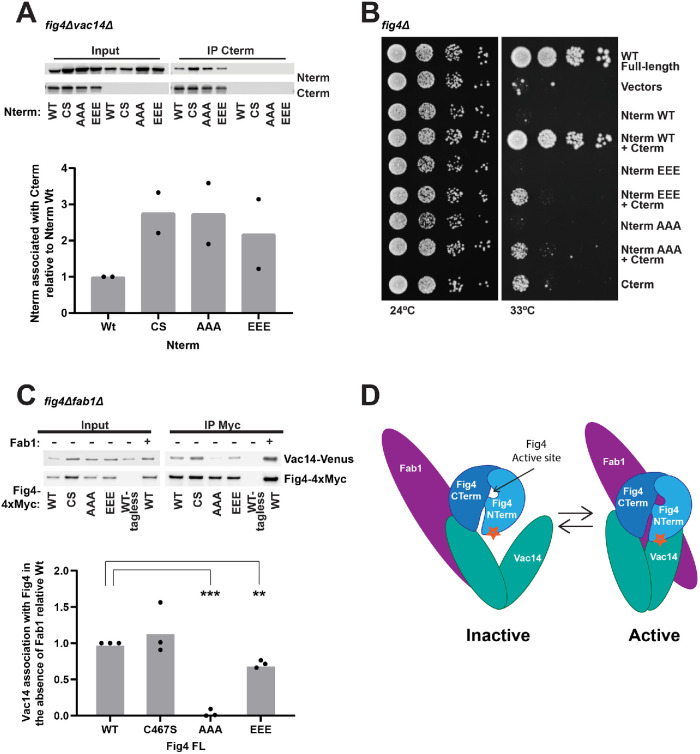
FIGURE 4:
The Fig4 N-terminal and C-terminal domains mediate Fab1-Vac14-Fig4 complex assembly and function through contacts on Vac14. (A) Western blot of proteins immunoprecipitated with anti-Myc antibody from a fig4Δvac14Δ strain overexpressing the Fig4 C-terminus (CTerm-4xMyc) and/or the wild-type (WT), C467S (CS), T52AT62AT78A (AAA), or T52ET62ET78E (EEE) Fig4 phosphatase domain (HA-N-term) via an ADH promoter. Bar graph shows quantification of band densities in Western blot, relative to wild type, normalized to Cterm-Myc with data points and mean from two independent experiments. (B) In contrast to wild type, Fig4-Nterm AAA and Nterm-EEE do not support growth in the presence of the Fig4-Cterm in fig4Δ Vac14-Venus Fab1-TAP at 33°C. Cells expressing no Fig4, Fig4-wild-type (WT), or overexpressing the indicated Nterm and Cterm constructs from plasmids were grown on selective Sc–his–leu plates at 24° and 33°C. (C) Western blot of proteins immunoprecipitated with anti-Myc antibody from a fig4Δfab1Δ expressing Fig4-WT, Fig4-CS, Fig4-AAA, or Fig4-EEE. Vac14-Venus expressed from endogenous locus. Bar graph shows quantification of band densities in Western blot, relative to wild type, normalized to Fig4-Myc, with data points and mean from three independent experiments (*** p < 0.0001, ** p < 0.001, two-tailed t test). (D) Model for regulation of the Fab1-Vac14-Fig4 complex. The Fig4 C-terminus and an N-terminal surface (star) both contribute to its association with Vac14 within the Fab1-Vac14-Fig4 complex. The Fig4 catalytic site is also required for the full activation of Fab1.