Abstract
The emergence of collagen I in vertebrates resulted in a dramatic increase in the stiffness of the extracellular environment, supporting long-range force propagation and the development of low-compliant tissues necessary for the development of vertebrate traits including pressurized circulation and renal filtration. Vertebrates have also evolved integrins that can bind to collagens, resulting in the generation of higher tension and more efficient force transmission in the extracellular matrix. The stiffer environment provides an opportunity for the vertebrates to create new structures such as the stress fibers, new cell types such as endothelial cells, new developmental processes such as neural crest delamination, and new tissue organizations such as the blood–brain barrier. Molecular players found only in vertebrates allow the modification of conserved mechanisms as well as the design of novel strategies that can better serve the physiological needs of the vertebrates. These innovations collectively contribute to novel morphogenetic behaviors and unprecedented increases in the complexities of tissue mechanics and functions.
INTRODUCTION
Three major driving forces in the evolution of vertebrates are the development of a stiffer extracellular environment, the emergence of collagen-binding integrins, and the significant increases in force generation in nonmuscle cells. These conditions, together with two rounds of whole genome duplications at the base of vertebrate evolution, give rise to new structures and cell types. The purpose of this Perspective is to speculate on how collagen I, stiffness, and adhesion impacted the evolution of mechanobiology, resulting in divergent mechanisms in vertebrates and nonvertebrates (Nakatani et al., 2007; Hufton et al., 2008; Hoffmann et al., 2012).
Multiple biological and animal models are used to study cellular behaviors such as tissue morphogenesis. However, in many instances, different systems produce conflicting observations and, in the field of mechanobiology, very different conclusions. These discrepancies are seen at many levels of investigations. At the tissue level, cell movement appears to result from different mechanisms during the development of Drosophila and Xenopus embryos (Keller, 1986; Keller et al., 1992; Keller and Winklbauer, 1992; Shih and Keller, 1992; Irvine and Wieschaus, 1994; Bertet et al., 2004; Zallen and Wieschaus, 2004; Shindo, 2018). At the signaling level, collective cell migration appears to be activated by distinct ERK-dependent mechanisms in Drosophila and mammalian cells (Aoki et al., 2017; Ogura et al., 2018). At the molecular level, different amounts of molecular force appear to be exerted at cell–cell adhesions in the developing Drosophila and zebrafish embryos (Yamashita et al., 2016; Eder et al., 2017; Lagendijk et al., 2017). Furthermore, the physiological roles of mechanosensitive molecules such as vinculin also appear to be different. For example, knockout of vinculin has little effect in Drosophila but is detrimental to zebrafish and mice (Alatortsev et al., 1997; Xu et al., 1998; Zemljic-Harpf et al., 2004; Cheng et al., 2016; Lausecker et al., 2018). In Drosophila, hyperactive vinculin inhibits the formation of integrin adhesion complexes, whereas the opposite is true for the mouse (Marg et al., 2010; Maartens et al., 2016). At the cellular level, novel cellular and molecular structures are found only in vertebrates. One novel structure found in nonmuscle cells is the stress fiber, which has an alternating actin and myosin II arrangement, analogous to the sarcomeres in muscle cells. The “sarcomere-like” actomyosin organization has not been described in nonvertebrate cells, although basal actin arrays and oriented actin bundles are found in Drosophila (Byers and Fujiwara, 1982; Drenckhahn and Wagner, 1986; Nehls and Drenckhahn, 1991; Delon and Brown, 2009; Cetera et al., 2014; Xie et al., 2014; Goodwin et al., 2016; Qin et al., 2017; Cerqueira Campos et al., 2020). Sarcomeric stress fibers are present at the apical junction of vertebrate epithelial cells but are not found at the Drosophila junction despite the significance of junctional actomyosin networks in intercellular movements (Fernandez-Gonzalez et al., 2009; Fernandez-Gonzalez and Zallen, 2011, 2013; Martin et al.,2010; Sawyer et al., 2011; Curran et al., 2017; Heuze et al., 2019). While such differences might reflect simple interspecies variability, it is also possible that much of mechanoregulation is not well-conserved between vertebrates and nonvertebrates (Stephenson et al., 2017; Soslau, 2020).
THICKER AND STIFFER FIBRILS INCREASE THE STIFFNESS OF EXTRACELLULAR MATRIX
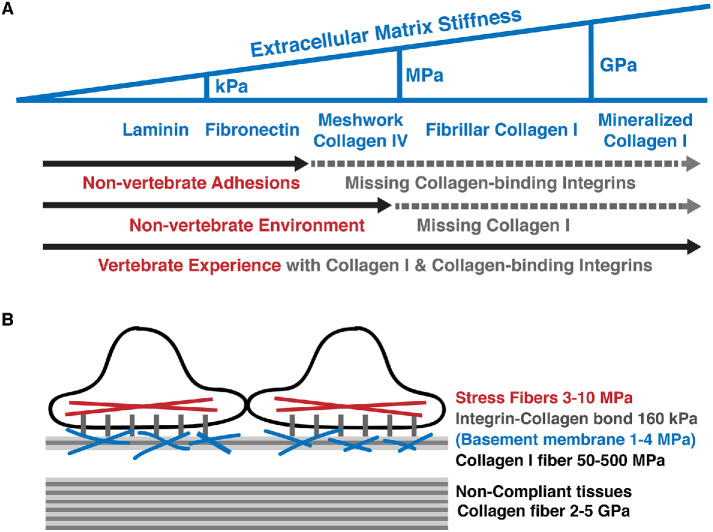
Collagen I is the primary component of the vertebrate extracellular matrix (ECM), making up ∼30% of the total protein mass in humans (Huxley-Jones et al., 2007; Kadler et al., 2007; Silver, 2009; Exposito et al., 2010). Collagen I forms thick, stiff, and long fibrils that are responsible for the dramatic decrease in tissue compliance in vertebrates (Silver et al., 2001, 2002; Ushiki, 2002; Boot-Handford and Tuckwell, 2003; Yang et al., 2008). Depending on the density and packing order of collagen I fibrils, vertebrates can construct ECM up to hundreds of megapascals in stiffness (van der Rijt et al., 2006; Candiello et al., 2007; Shen et al., 2008, 2011; Yang et al., 2008; Svensson et al., 2010; Aifantis et al., 2011; Kohn et al., 2015); Mineralization of collagen I fibers further increases the stiffness of the matrix to several gigapascals, a magnitude that is orders of magnitude higher than the ECMs than most nonvertebrates would have ever experienced (Figure 1A) (Kawasaki et al., 2004; Landis and Silver, 2009; Chlasta et al., 2017).
FIGURE 1:
A reductionist’s view of the extracellular experience in vertebrates and nonvertebrates. (A) The evolutionarily conserved fibronectin, collagen IV, and laminin form meshwork-type matrixes with stiffnesses up to hundreds of kilopascals (kPa). In vertebrates, collagen I forms matrixes with stiffnesses ranging from megapascals (MPa) to gigapascals (GPa). Nonvertebrates and vertebrates can bind to laminin and fibronectin, whereas only vertebrates interact directly with collagen (see the text for details). (B) Two cells are interacting with the ECM and with each other. Force generated by stress fibers inside the cell can be transmitted to the extracellular collagen matrix via collagen-binding integrins. A basement membrane containing a mixture of matrix proteins is present in some specialized tissues.
CONNECTING ECM TO CELLS
The major force-transmission pathway connecting the force-generating actomyosin structures inside the cells to the ECM occurs via cell surface integrins (Figure 1B) (Niland et al., 2011; Livne and Geiger, 2016; Carey et al., 2017). Vertebrate and nonvertebrate cells can interact with the evolutionarily more conserved fibronectin and laminin through fibronectin-binding and laminin-binding integrins. However, only vertebrates have evolved collagen-binding integrins to interact with collagens. Thus, despite the presence of collagen IV in all metazoa, nonvertebrate cells do not interact directly with collagen IV because they do not have the collagen-binding integrins (Ewan et al., 2005; Chouhan et al., 2014; Johnson and Chouhan, 2014).
In vertebrates, fibronectin can coassemble with collagen I to form fibers that potentially can interact with both fibronectin-binding and collagen-binding integrins concomitantly (Kadler et al., 2008; Singh et al., 2010; Kubow et al., 2015; Lemmon and Weinberg, 2017; Mezzenga and Mitsi, 2019). Thus, the emergence of collagen I not only increases the stiffness of the overall extracellular environment, it also alters the rigidity and organization of the fibronectin network, allowing a softer meshwork-type matrix to behave as a stiffer fibrillar-type substrate. This has far-reaching implications and consequences because fibronectin-binding integrins have mechanotransduction roles and can sense the stiffness of the fibronectin matrix (Tee et al., 2011; Trichet et al., 2012; Carraher and Schwarzbauer, 2013; Ribeiro et al., 2014; van Geemen et al., 2014; Zhou et al., 2017; Janmey et al., 2020). By dramatically increasing the stiffness and organization of the fibronectin matrix, collagen I supports a wider range of mechanotransduction responses elicited by fibronectin-binding integrins.
COLLAGEN-BINDING INTEGRINS FORM THE STRONGEST CELL–ECM BONDS
The emergence of collagen-binding integrins is one of the most important events in the evolution of vertebrate mechanobiology (Calderwood et al., 1995; Tuckwell et al., 1995; Tulla et al., 2001; Jokinen et al., 2004; Ewan et al., 2005; Chouhan et al., 2014; Johnson and Chouhan, 2014). Collagen-binding integrins form bonds with collagen that are stronger than any integrin–fibronectin or integrin–laminin bonds, and thus are the load-bearing structures for the vertebrate cell-matrix adhesion (Tiger et al., 2001; Chen et al., 2004; Huhtala et al., 2005; Zhang et al., 2006; Louis et al., 2007; Kong et al., 2009; Roca-Cusachs et al., 2009, 2012; Carracedo et al., 2010; Hu et al., 2011; Niland et al., 2011; Popov et al., 2011; Zeltz and Gullberg, 2016; Ciuba et al., 2018). The direct coupling between integrin and collagen plays a significant role in the generation of higher tension and more efficient force transmission in the collagen matrix (Hu et al., 2011; Mohammadi et al., 2015b). Furthermore, the ability to exert force directly on collagen fibers allows the acquisition of novel cellular behaviors including multicellular streaming and contact guidance (Tiger et al., 2001; Tamariz and Grinnell, 2002; Wolf et al., 2003, 2009; Miron-Mendoza et al., 2010; Carey et al., 2016; Grossman et al., 2016; Han et al., 2016; Nuhn et al., 2018; Sarker et al., 2019). Collagen-binding integrins and fibronectin-binding integrins form discrete molecular complexes and have distinct mechanotransduction functions, contributing to cell-matrix biology in complementary ways in vertebrates (Ylanne et al., 1993; Hocking et al., 2000; Teravainen et al., 2013; Ribeiro et al., 2014; Burridge and Guilluy, 2016; Roca-Cusachs et al., 2013).
COLLAGEN I SUPPORTS LONG-RANGE FORCE TRANSMISSION
Vertebrate collagen I has evolved new biochemical strategies to form compact, staggered, and covalently cross-linked fibrils that are thicker, stiffer, and longer than any ancestral fibrillar or meshwork collagens (Pins et al., 1997; Eyre and Wu, 2005; Ricard-Blum, 2011; Kwansa et al., 2016). Collagen I is more efficient for directional force propagation due to the increased fiber length, the higher rigidity, and the precise alignment of collagen I molecules in the fibers. The amount of force that can be generated in collagen I matrixes is substantially higher, up to several orders of magnitude, than that that can be supported by fibronectin, laminin, or collagen IV meshwork-type matrixes (Hocking et al., 2000; Boot-Handford and Tuckwell, 2003; Candiello et al., 2007; Dominguez-Gimenez et al., 2007; Araki et al., 2009; Silver, 2009; Exposito et al., 2010; Maruthamuthu et al., 2011; Sztal et al., 2011; Tee et al., 2011; Fahey and Degnan, 2012; Trichet et al., 2012; Roca-Cusachs et al., 2013; Teravainen et al., 2013; Cetera et al., 2014; Xie et al., 2014; Adams et al., 2015; Kubow et al., 2015; Burridge and Guilluy, 2016; Goodwin et al., 2016; Chlasta et al., 2017; Fidler et al., 2017, 2018; Filla et al., 2017; Qin et al., 2017; Sancho et al., 2017; Shook et al., 2018; Zollinger et al., 2018; Draper et al., 2019; Cerqueira Campos et al., 2020; Chronopoulos et al., 2020).
How exactly might collagen I bring new mechanobiology to vertebrates? At least four factors are important (Figure 2):
(1) The number of binding sites matters. The number of integrin-binding sites on a matrix molecule determines whether it can cluster the integrins and how much force it can transmit (Figure 2A). A laminin trimer interacts with only one integrin, whereas a fibronectin dimer has two integrin-binding sites. A collagen fibril contains tens to hundreds of covalently cross-linked molecules, providing many integrin-binding sites (Xu et al., 2000; Pichard et al., 2001; Turner et al., 2020). In vivo, fibronectin can form larger assemblies via noncovalent interactions, increasing the apparent binding sites. Collagen IV can also form large assemblies via noncovalent interactions and disulfide bonds. However, these networks are deformable and can buckle, collapse, or rupture when stretched. On the other hand, the extensively cross-linked collagen fiber behaves as one unit under force. Thus, the total amount of force that can be applied to a collagen fiber is substantially higher than the forces that can be applied to meshwork-type matrixes. Multiple interactions between integrins and the collagen fiber would allow force integration necessary for stiffness sensing by the cells (Jiang et al., 2016; Janmey et al., 2020).
(2) Size and length matters. The nonmuscle myosin II minifilament, which is ∼300 nm long, forms the force-generating unit of the stress fiber (Pellegrin and Mellor, 2007; Billington et al., 2013; Dasbiswas et al., 2018). To generate tension at integrin adhesions, two integrins must be positioned at least as far apart as the length of a myosin II minifilament. A fibronectin dimer has a length of ∼100 nm, and while it can cluster two integrins it cannot support tension generation. Thus, contraction of the stress fiber will pull on both integrins, dragging the fibronectin dimers and the integrins in the same direction. If the fibronectin dimers are isolated, there will be no resistance to the movement and no tension will be generated within the matrix (Figure 2B). In a fibronectin network, pulling on fibronectin would lead to stretching of the network. However, if the pulling force exceeds the strength of the noncovalent bonds, the fibronectin network could break, interrupting the force-transmission pathway and tension generation. Collagen IV meshworks are held together by disulfide bonds and noncovalent interactions and thus can withstand pulling force when stretched, possibly permitting tension to build up in the distorted matrix (Figure 2C). In vertebrates, a collagen I fiber can be up to tens of microns in length and thus can integrate many “sarcomere-like” contractile units, increasing the force exerted at individual cell-matrix adhesions and resulting in greater tension to be generated within the ECM. When two noncontacting cells are bound to the same long collagen I fiber, pulling force from one cell can be sensed by a neighboring cell (Figure 2D). Traction force generated at collagen-binding integrins can realign or translocate the collagen I fiber, resulting in the remodeling of the ECM (Ramos and DeSimone, 1996; Davidson et al., 2002, 2004, 2008; Marsden and DeSimone, 2003; Miron-Mendoza et al., 2008; Wang et al., 2014; Han et al., 2018; Ban et al., 2019; Shiflett et al., 2019).
(3) Stiffness matters. Matrixes composed of fibronectin, laminin, and collagen IV form meshworks that are stretchable by cellular force, whereas collagen I is long and compact fibers that are stiff and relatively unbendable by cellular force. The propagation of force within fibronectin and collagen IV meshworks primarily goes through noncovalent protein–protein interfaces. In the presence of pulling force that can break noncovalent bonds, meshwork-type matrixes will fail to transmit force while the covalently cross-linked collagen I fiber will continue to support force propagation. In vertebrates, the interaction of fibronectin with fibrillar collagen I via noncovalent bonds could alter the apparent biophysical properties and stiffness of the fibronectin network. However, fibronectin is extremely distensible and unfolds almost completely under physiological force, reaching a tensile stiffness of only 1–2 MPa when maximally stretched, which is at least two orders of magnitude lower than the tensile stiffness of collagen I fibrils (van der Rijt et al., 2006; Shen et al., 2008; Yang et al., 2008; Klotzsch et al., 2009; Svensson et al., 2010; Bradshaw and Smith, 2014). Moreover, fibronectin–integrin bonds are not as strong as collagen–integrin bonds. Therefore, force propagation in collagen I matrix is much more efficient due to the covalent intermolecular bonds, the higher tensile stiffness of the collagen I fibers, and the stronger bonds between collagen and collagen-binding integrins. In this regime, higher levels of tension can be accumulated within the ECM. The tensile energy in the stiffer matrix, in turn, would increase the stiffness of the cell and promote greater force generation that can be used to assemble stress fibers, remodel focal adhesions, promote cell–cell adhesions, and ultimately increase the overall potential energy of the tissue (Walcott and Sun, 2010; Maruthamuthu et al., 2011; Wolfenson et al., 2011; DeMali et al., 2014; Wang et al., 2014; Wong et al., 2014; Ye et al., 2014; Broaders et al., 2015; Ronan et al., 2015; Hall et al., 2016; Hu et al., 2017; Le et al., 2017; Dasbiswas et al., 2018; Han et al., 2018; Kuragano et al., 2018b; Chang et al., 2019).
(4) Geometry matters. Collagen IV and fibronectin form meshworks that are isotropic and would dissipate force in all directions, thus cannot faithfully reproduce the vector quality of force (Figure 2A). In contrast, collagen I fibrils behave like linear cables that can support the propagation of directional information, alignment of stress fibers, and the asymmetric growth of focal adhesions (Figure 2D). Importantly, linear collagen I fibers provide physical cues for directed protrusions and cell migration (Kaunas et al., 2005; Besser and Safran, 2006; Miron-Mendoza et al., 2010; Foolen et al., 2014; Tondon and Kaunas, 2014; Mohammadi et al., 2015a; Han et al., 2016; Xie et al., 2017; Brauer et al., 2019; Sarker et al., 2019).
FIGURE 2:
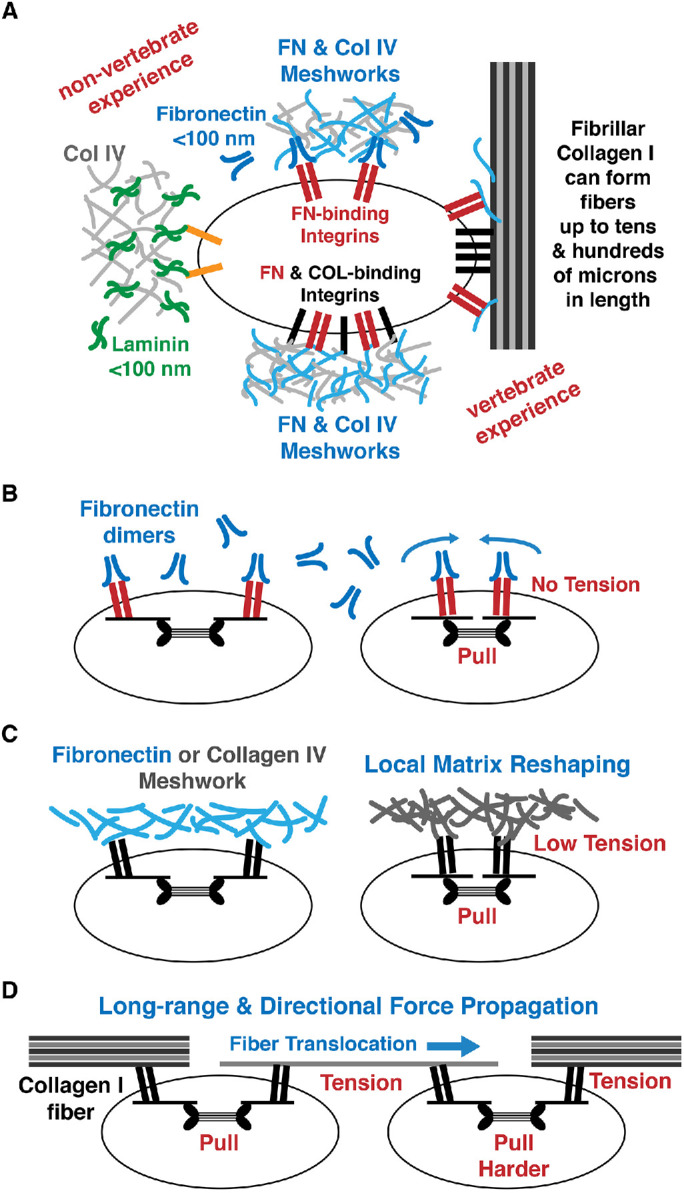
Collagen I forms stiffer, thicker, and longer fibers to support the generation of tension and long-range force propagation. (A) In nonvertebrates, cells can interact with laminin and fibronectin (FN) via laminin-binding and fibronectin-binding integrins. However, nonvertebrates do not have collagen-binding integrins and cannot exert force on the collagen (COL) IV matrix. Vertebrate cells have both fibronectin-binding and collagen-binding integrins and can bind directly to fibronectin and collagens (see the text for details). (B) Pulling on fibronectin-binding integrins that are bound to fibronectin dimers results in movement of the integrins with no change in tension in the matrix. (C) Pulling on collagen-binding integrins will generate tension in collagen IV meshworks (see the text for details). Pulling on a distensible fibronectin network may also result in the generation of tension in the matrix (see the text for details). (D) Long-distance force transmission and tension generation is supported by collagen I fiber attached to two cells via collagen-binding integrins. Pulling a collagen fiber by a cell can translocate the fiber.
THICKER, STIFFER, LONGER FIBRILS SUPPORT HIGHER FORCE PRODUCTION
Vertebrate cells stiffen when attached to collagen I, exerting forces up to hundreds of nanonewtons which is nearly an order of magnitude higher than that can be generated by fibronectin-attached cells (Hocking et al., 2000; Araki et al., 2009; Maruthamuthu et al., 2011; Tee et al., 2011; Trichet et al., 2012; Roca-Cusachs et al., 2013; Teravainen et al., 2013; Kubow et al., 2015; Burridge and Guilluy, 2016; Sancho et al., 2017; Zollinger et al., 2018; Chronopoulos et al., 2020). Experimental evidence indicates that the maturation process of fibronectin-based adhesions is independent of force (Oakes et al., 2012; Stricker et al., 2013), raising the possibility that fibronectin-based adhesions are fundamentally different from collagen-based adhesions.
There are many possible ways to increase force production at collagen-based adhesions in vertebrates:
(1) Turn on myosin II ATPase. One way to increase force production is to activate pathways that phosphorylate and activate myosin II and turn off pathways that dephosphorylate and inactivate myosin II (Tojkander et al., 2012; Ciuba et al., 2018). The molecular motor myosin II is responsible for force generation in cells and is regulated by the phosphorylation status of the myosin light chain. In cells, myosin light chain is phosphorylated by myosin kinase. Thus, activating signals such as Rho, Rock, and calcium-calmodulin that increase the activities of myosin kinase would stimulate myosin contraction. Myosin phosphatase, in turn, dephosphorylates the myosin regulatory light chain and ends contraction. Activating signaling pathways that inhibit myosin phosphatase will promote myosin light chain phosphorylation and sustained contractility.
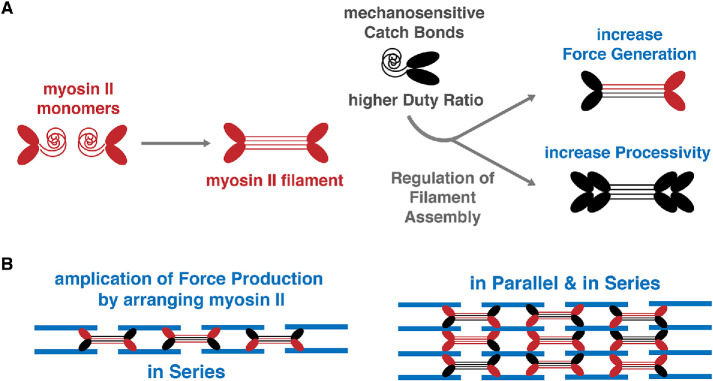
(2) Modify core machinery. To increase force production, myosin II assemblies with more motor heads can be formed by regulating the stability and the assembly of myosin II minifilaments. Increasing the duty ratio of the myosin motor would increase the duration of myosin interaction with actin, thus increasing the processivity of individual motors. In addition, altering the mechanoresponse of the myosin motor would increase the strength of the myosin–actin bond under mechanical stress. These properties are already implemented by the vertebrate-specific myosin IIB (Figure 3A), a paralogue of the ancestrally derived myosin IIA (Murakami et al., 1998, 2000; Chantler et al., 2010; Stam et al., 2015; Heissler and Sellers, 2016; Kuragano et al., 2018a; Melli et al., 2018).
(3) Build new structures using existing components. Each myosin II minifilament is capable of producing force of ∼30–50 piconewtons, but when the cells are attached to the collagen I matrix, they can generate forces up to hundreds of nanonewtons (Kaya and Higuchi, 2010; Sim et al., 2015; Sancho et al., 2017). This means that thousands of myosin II minifilaments must work synergistically to produce the amount of force that is measured at collagen I–based adhesions. To amplify force production, the cell organizes the myosin minifilaments and arranges them in parallel and in series, rather than randomly positioning them inside the cytoplasm (Figure 3B). Indeed, this organization is used in vertebrate nonmuscle cells to build actomyosin stress fibers capable of generating force that is an order of magnitude higher than the sum produced by individual myosin motors (Pellegrin and Mellor, 2007; Lohner et al., 2019).
FIGURE 3:
Controlling force production by myosin II regulation. (A) Force generation by stress fibers can be modified by increasing the duty ratio of the myosin II motor, processivity of the myosin II minifilament, mechanosensitivity of the myosin molecule, and the size of the myosin minifilament. (B) Force production by myosin II minifilaments can be amplified by arranging them in series and in parallel.
Forces generated by stress fibers are transmitted to the ECM through focal adhesions, which are sites of primary traction consisting of clustered integrins and strengthened linkage to actin (Ye et al., 2014; Burridge and Guilluy, 2016; Livne and Geiger, 2016). Focal adhesions formed by collagen-binding integrins are essential for vertebrate mechanobiology by participating in stiffness sensing, cell migration, and remodeling of the ECM (Burridge and Fath, 1989; Fath et al., 1989; Plotnikov et al., 2012; Bays et al., 2014; Hall et al., 2016; Chang et al., 2019; Puleo et al., 2019).
TUNING FORCE IN A STIFFER ENVIRONMENT SUPPORTED BY WHOLE GENOME DUPLICATIONS
To manage the new biology that comes with a stiffer environment, vertebrates are provided with a new toolbox, brought in part by a major evolutionary event consisting of two rounds of whole genome duplications (Vandepoele et al., 2004; Dehal and Boore, 2005; Holland and Ocampo Daza, 2018; Marletaz et al., 2018). This major evolutionary event gives rise to paralogues at a genomic scale that marks the branching of vertebrates from the invertebrates ∼500–550 million years ago. Thus, a significant percentage of adhesion and cytoskeletal proteins in vertebrates have multiple copies, one from the ancestral gene and the others from duplicated genes, for example, filamin A-C, paxillin α-γ, myosin IIA-C, α-actinin-1-4, tropomyosin1-4, WAVE 1-3, RhoA-C, Rac1-3, and talin1-2 (Boureux et al., 2007; Gehler et al., 2009; Kurisu and Takenawa, 2009; Rahimzadeh et al., 2011; Billington et al., 2013; Austen et al., 2015; Mohammadi et al., 2015b; Meacci et al., 2016; Schiffhauer et al., 2016; Kuragano et al., 2018a; Pathan-Chhatbar et al., 2018; Puleo et al., 2019; Sao et al., 2019). While the ancestral copy often retains its original function, the duplicated copies frequently acquire new functions due to the lack of selection pressures on the copies. The expanded protein toolbox provides new options for the vertebrates to increase complexity and evolve novel molecular structures, mechanisms, and regulations (Brady et al., 2009; Laurin et al., 2019).
One way to build complexity into mechanoregulation is to adjust force production and tune mechanoresponses. Vertebrates have many options to handle this challenge:
(1) Assign new functions to an ancestral protein that has no essential function in nonvertebrates. For example, vinculin is expendable in Drosophila but, in vertebrates, it plays an important role in the strengthening of cell–cell and cell–matrix adhesions under mechanical stress (Alatortsev et al., 1997; Cheng et al., 2016; Bays and DeMali, 2017; Le et al., 2019).
(2) Evolve new proteins and structures that are absent in nonvertebrates. Many newly emerged vertebrate proteins and structures are designed to work with conserved molecules and core mechanisms to increase complexity (Mariani et al., 2020). One example is the intermediate filament vimentin, which localizes to focal adhesions and regulates the assembly of integrin adhesion complexes under mechanical stress (Tsuruta and Jones, 2003; Sanghvi-Shah and Weber, 2017). Another example is the tight junction, which can modulate the mechanical input at the adherens junction in epithelial cells (Hatte et al., 2018).
(3) Subfunctionalize paralogues to tune force production. Force generation can be adjusted by controlling stress fiber dynamics or focal adhesion stability. One example of subfunctionalization is the differential use of SORBS family members to control the amount of force applied to the ECM (Kuroda et al., 2018). While the vertebrate paralogue SORBS2 interacts with α-actinin to regulate stress fiber contractility, the ancestral paralogue SORBS1 interacts with vinculin to control focal adhesion maturation (Ichikawa et al., 2017). Another example is the differential usage of myosin IIA and IIB paralogues, resulting in the spatial regulation of stress fiber formation, front–back polarity, ECM remodeling, and intercellular junction dynamics (Even-Faitelson and Ravid, 2006; Vicente-Manzanares et al., 2007; Sandquist and Means, 2008; Solinet and Vitale, 2008; Smutny et al., 2010; Doyle et al., 2012; Gutzman et al., 2015; Stam et al., 2015; Ridge et al., 2017; Kim et al., 2018; Kuragano et al., 2018a, b; Heuze et al., 2019).
A COMPLEX EXPERIENCE FOR THE VERTEBRATE CELL
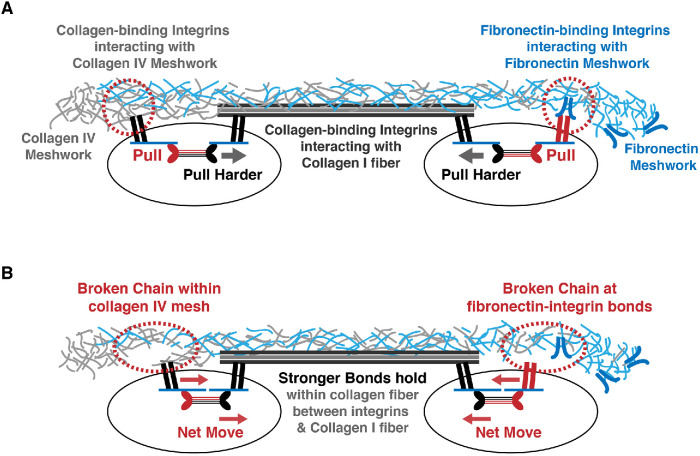
The expanded toolbox, along with collagen I and collagen-binding integrins, created exceptional opportunities for the vertebrates to build complexities in mechanobiology (Figure 4, A and B). To illustrate, two cells were drawn in Figure 4A; the cell on the left has a myosin II minifilament that is composed of myosin IIA (red motor heads) and IIB (black motor heads). The hybrid myosin IIA/IIB minifilament is attached to two focal adhesions containing collagen-binding integrins; one focal adhesion is bound to collagen IV meshwork (red dotted circle on the left cell), and the other focal adhesion is bound to a collagen I fiber. Myosin IIB pulls harder than myosin IIA and moves the minifilament toward the collagen I fiber, exerting force on the collagen IV meshwork and ultimately breaking the noncovalent bonds within the collagen IV matrix (Figure 4B, red dotted oval on the left cell). Using the same argument, the cell on the right in Figure 4A has a hybrid myosin II minifilament composed of myosin IIA (red motor heads) and IIB (black motor heads). The stress fiber is attached to two focal adhesions; one focal adhesion is bound to the fibronectin meshwork via fibronectin-binding integrins (red dotted circle on the right cell), and the other focal adhesion is bound to a collagen I fiber via collagen-binding integrins. Myosin IIB wins the tug-of-war, and the asymmetric hybrid myosin IIA/IIB minifilament moves toward the collagen I fiber, pulling on the fibronectin-binding integrins and breaking their interaction with the fibronectin matrix (Figure 4B, red dotted oval on the right cell). Thus, the weakest link in the chain dictates the upper limit of force transmission (Burridge and Guilluy, 2016). This concept is central to mechanobiology and holds the biophysical basis for mechanoregulatory principles and models.
FIGURE 4:
The weakest-link-in-the-chain concept in force transmission (Burridge and Guilluy, 2016). (A) Example of a complex cellular experience involving two cells embedded in an extracellular environment composed of collagen IV, collagen I, and fibronectin. The two cells are expressing different combinations of fibronectin-binding and collagen-binding integrins on their cell surfaces. A collagen fiber is interacting with both cells. In the cell on the left, two focal adhesions with collagen-binding integrins are interacting with two different matrixes; one focal adhesion is bound to a collagen IV meshwork (red dotted circle on the left cell), and the other focal adhesion is bound to a collagen I fiber. A hybrid minifilament composed of myosin IIA (red motor heads) and IIB (black motor heads) is attached to the stress fiber linking the two focal adhesions. In the cell on the right, the hybrid myosin IIA/IIB minifilament is attached to two focal adhesions consisting of different integrins; one focal adhesion is bound to the fibronectin meshwork via fibronectin-binding integrins (red dotted circle on the right cell), and the other focal adhesion is bound to a collagen I fiber via collagen-binding integrins. (B) Contraction force from the stress fibers can differentially regulate cell-matrix adhesions and extracellular force transmission in the matrix. In the cell on the left, myosin IIB pulls harder than myosin IIA and moves the minifilament toward the collagen I fiber, exerting force on the collagen IV meshwork and ultimately breaking the noncovalent bonds within the collagen IV matrix (red dotted oval on the left cell). In the cell on the right, myosin IIB wins the tug-of-war and the myosin IIA/IIB minifilament moves toward the collagen I fiber, pulling on the fibronectin-binding integrins and breaking their interaction with the fibronectin matrix (red dotted oval on the right cell).
CONCLUDING REMARKS
The emergence of collagen I drastically changed the compliance, porosity, stiffness, and organization of the ECM in vertebrate tissues. However, changing the biophysical properties of the extracellular environment alone is not sufficient for the evolution of new processes unless the stiffer matrix is linked to the cell. This is achieved by the coemergence of collagen-binding integrins at the base of the vertebrate evolution ∼550 million years ago, resulting in a powerful change in the energy landscape in cells and in tissues. Ultimately, the dawn of vertebrate mechanobiology is realized by two rounds of whole genome duplications, providing new tools for regulation, amplification, and integration.
The decrease in tissue compliance allows the development of blood pressures to support pressurized renal filtration, a vertebrate invention that is superior in design for the management of electrolytes and metabolites (Schulte et al., 2015; Stephenson et al., 2017; Soslau, 2020). The increase in hydrostatic pressures in the closed circulatory system of the vertebrates necessitates the development of a vascular lining to prevent transmural leakage of vascular contents. The emergence of endothelial cells that line the vascular wall is another significant vertebrate innovation that coemerged with collagen I and collagen-binding integrins. Endothelial cells are equipped with specialized cell–cell junctions to act as permeability barriers and have strengthened cell–cell adhesions to resist hydraulic mechanical stress. The plasticity of the endothelial cells allows functional specialization of the endothelium, including a system of low-pressure capillary beds for nutrient delivery and the formation of the blood–brain barrier (Bundgaard and Abbott, 2008; O’Brown et al., 2018). The morphogenesis and the development of the endothelial vasculature, a process known as angiogenesis, is dependent on collagen-binding integrins on the endothelial cells and is supported by the collagen I ECM (Senger et al., 1997; Sweeney et al., 2003; Whelan and Senger, 2003; San Antonio et al., 2009; Kick et al., 2016; Post et al., 2019; Turner et al., 2020).
The endothelium ushers the appearance of multiple vertebrate-specific organs that are the footprints and signatures of vertebrate evolution (Shigei et al., 2001; Vize and Smith, 2004; Munoz-Chapuli et al., 2005; Monahan-Earley et al., 2013). Endothelial cells are essential for the development of the vertebrate liver, pancreas, lungs, and kidneys during embryo morphogenesis. In adult animals, endothelial cells play significant roles in the physiological functions of the kidneys to concentrate urine and the lungs to perform gaseous exchange (Lammert et al., 2003; Cleaver and Dor, 2012; Ramasamy et al., 2015; Rafii et al., 2016; Bastidas-Ponce et al., 2017; Daniel and Cleaver, 2019). The morphogenesis of these vertebrate-specific organs is dependent on the proper deposition and organization of collagen I (Aycock and Seyer, 1989; Goldstein, 1991; Saelman et al., 1995; Shimeld and Holland, 2000; Chen et al., 2004; Riopel and Wang, 2014; Buchtler et al., 2018; Stephens et al., 2018).
Endothelial cells also play a significant role in the development of neural crest derivatives including the vertebrate skull, the brachial skeleton, and the sensory ganglia. Endothelial cells interact with neural crest cells, another key innovation of the vertebrates, and influence the distribution, the migratory pathways, and the patterned formation of neural crest cell derivatives. Neural crest migration from their origin of formation between the neural plate and the surface ectoderm is dependent on collagen I and requires interaction with the matrix via collagen-binding integrins (Duband and Thiery, 1987; Perris et al., 1991; Nagy et al., 2009; Diogo et al., 2015; George et al., 2016).
Further along the evolutionary timeline, mammals continue to utilize fibrillar collagens and collagen-binding integrins to invent new mechanosensitive processes, structures, and physiology, including the development of the mammalian placenta and invasion of the uterine wall by trophoblasts in a stiffness-dependent manner during embryo implantation (Damsky et al., 1994; Braasch et al., 2009; Yoshida et al., 2014; Griffith and Wagner, 2017; Park et al., 2017; Abbas et al., 2019; Zambuto et al., 2019). In conclusion, vertebrate mechanobiology is created, in part, by three major evolutionary forces including collagen I, collagen-binding integrins, and vertebrate-specific proteins to support the development of novel molecular mechanisms, adding to the repertoire of mechanisms that is already in place and shared by nonvertebrates and vertebrates (Rozario and DeSimone, 2010).
Acknowledgments
I thank Alpha Yap for bringing up the idea of writing this commentary at ASCB last year. I also thank Bill Brieher for his challenging skepticisms on the topic that forces me to look for answers. Funding is provided by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (R01 DK098398) to V.W.T.
Abbreviations used:
- COL
collagen
- ECM
extracellular matrix
- FN
fibronectin
- GPa
gigapascals
- kPa
kilopascals
- MPa
megapascals
Footnotes
REFERENCES
- Abbas Y, Carnicer-Lombarte A, Gardner L, Thomas J, Brosens JJ, Moffett A, Sharkey AM, Franze K, Burton GJ, Oyen ML. (2019). Tissue stiffness at the human maternal-fetal interface. Hum Reprod , 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Chiquet-Ehrismann R, Tucker RP. (2015). The evolution of tenascins and fibronectin. Cell Adh Migr , 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aifantis KE, Shrivastava S, Odegard GM. (2011). Transverse mechanical properties of collagen fibers from nanoindentation. J Mater Sci Mater Med , 1375–1381. [DOI] [PubMed] [Google Scholar]
- Alatortsev VE, Kramerova IA, Frolov MV, Lavrov SA, Westphal ED. (1997). Vinculin gene is non-essential in Drosophila melanogaster. FEBS Lett , 197–201. [DOI] [PubMed] [Google Scholar]
- Aoki K, Kondo Y, Naoki H, Hiratsuka T, Itoh RE, Matsuda M. (2017). Propagating wave of ERK activation orients collective cell migration. Dev Cell , 305–317.e305. [DOI] [PubMed] [Google Scholar]
- Araki E, Momota Y, Togo T, Tanioka M, Hozumi K, Nomizu M, Miyachi Y, Utani A. (2009). Clustering of syndecan-4 and integrin beta1 by laminin alpha 3 chain-derived peptide promotes keratinocyte migration. Mol Biol Cell , 3012–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen K, Ringer P, Mehlich A, Chrostek-Grashoff A, Kluger C, Klingner C, Sabass B, Zent R, Rief M, Grashoff C. (2015). Extracellular rigidity sensing by talin isoform-specific mechanical linkages. Nat Cell Biol , 1597–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aycock RS, Seyer JM. (1989). Collagens of normal and cirrhotic human liver. Connect Tissue Res , 19–31. [DOI] [PubMed] [Google Scholar]
- Ban E, Wang H, Franklin JM, Liphardt JT, Janmey PA, Shenoy VB. (2019). Strong triaxial coupling and anomalous Poisson effect in collagen networks. Proc Natl Acad Sci USA , 6790–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas-Ponce A, Scheibner K, Lickert H, Bakhti M. (2017). Cellular and molecular mechanisms coordinating pancreas development. Development , 2873–2888. [DOI] [PubMed] [Google Scholar]
- Bays JL, DeMali KA. (2017). Vinculin in cell-cell and cell-matrix adhesions. Cell Mol Life Sci , 2999–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays JL, Peng X, Tolbert CE, Guilluy C, Angell AE, Pan Y, Superfine R, Burridge K, DeMali KA. (2014). Vinculin phosphorylation differentially regulates mechanotransduction at cell-cell and cell-matrix adhesions. J Cell Biol , 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature , 667–671. [DOI] [PubMed] [Google Scholar]
- Besser A, Safran SA. (2006). Force-induced adsorption and anisotropic growth of focal adhesions. Biophys J , 3469–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington N, Wang A, Mao J, Adelstein RS, Sellers JR. (2013). Characterization of three full-length human nonmuscle myosin II paralogs. J Biol Chem , 33398–33410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot-Handford RP, Tuckwell DS. (2003). Fibrillar collagen: the key to vertebrate evolution? A tale of molecular incest. Bioessays , 142–151. [DOI] [PubMed] [Google Scholar]
- Boureux A, Vignal E, Faure S, Fort P. (2007). Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol , 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I, Volff JN, Schartl M. (2009). The endothelin system: evolution of vertebrate-specific ligand-receptor interactions by three rounds of genome duplication. Mol Biol Evol , 783–799. [DOI] [PubMed] [Google Scholar]
- Bradshaw MJ, Smith ML. (2014). Multiscale relationships between fibronectin structure and functional properties. Acta Biomater , 1524–1531. [DOI] [PubMed] [Google Scholar]
- Brady DC, Alan JK, Madigan JP, Fanning AS, Cox AD. (2009). The transforming Rho family GTPase Wrch-1 disrupts epithelial cell tight junctions and epithelial morphogenesis. Mol Cell Biol , 1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer E, Lippens E, Klein O, Nebrich G, Schreivogel S, Korus G, Duda GN, Petersen A. (2019). Collagen fibrils mechanically contribute to tissue contraction in an in vitro wound healing scenario. Adv Sci (Weinh) , 1801780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broaders KE, Cerchiari AE, Gartner ZJ. (2015). Coupling between apical tension and basal adhesion allow epithelia to collectively sense and respond to substrate topography over long distances. Integr Biol (Camb) , 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtler S, Grill A, Hofmarksrichter S, Stockert P, Schiechl-Brachner G, Rodriguez Gomez M, Neumayer S, Schmidbauer K, Talke Y, Klinkhammer BM, et al. (2018). Cellular origin and functional relevance of collagen I production in the kidney. J Am Soc Nephrol , 1859–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M, Abbott NJ. (2008). All vertebrates started out with a glial blood-brain barrier 4—500 million years ago. Glia , 699–708. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K. (1989). Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. Bioessays , 104–108. [DOI] [PubMed] [Google Scholar]
- Burridge K, Guilluy C. (2016). Focal adhesions, stress fibers and mechanical tension. Exp Cell Res , 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers HR, Fujiwara K. (1982). Stress fibers in cells in situ: immunofluorescence visualization with antiactin, antimyosin, and anti-alpha-actinin. J Cell Biol , 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA, Tuckwell DS, Humphries MJ. (1995). Specificity of integrin I-domain-ligand binding. Biochem Soc Trans , 504S. [DOI] [PubMed] [Google Scholar]
- Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, Lin H. (2007). Biomechanical properties of native basement membranes. FEBS J , 2897–2908. [DOI] [PubMed] [Google Scholar]
- Carey SP, Goldblatt ZE, Martin KE, Romero B, Williams RM, Reinhart-King CA. (2016). Local extracellular matrix alignment directs cellular protrusion dynamics and migration through Rac1 and FAK. Integr Biol (Camb) , 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey SP, Martin KE, Reinhart-King CA. (2017). Three-dimensional collagen matrix induces a mechanosensitive invasive epithelial phenotype. Sci Rep , 42088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo S, Lu N, Popova SN, Jonsson R, Eckes B, Gullberg D. (2010). The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem , 10434–10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraher CL, Schwarzbauer JE. (2013). Regulation of matrix assembly through rigidity-dependent fibronectin conformational changes. J Biol Chem , 14805–14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira Campos F, Dennis C, Alegot H, Fritsch C, Isabella A, Pouchin P, Bardot O, Horne-Badovinac S, Mirouse V. (2020). Oriented basement membrane fibrils provide a memory for F-actin planar polarization via the dystrophin-dystroglycan complex during tissue elongation. Development , dev186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetera M, Ramirez-San Juan GR, Oakes PW, Lewellyn L, Fairchild MJ, Tanentzapf G, Gardel ML, Horne-Badovinac S. (2014). Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat Commun , 5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SS, Rape AD, Wong SA, Guo WH, Wang YL. (2019). Migration regulates cellular mechanical states. Mol Biol Cell , 3104–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler PD, Wylie SR, Wheeler-Jones CP, McGonnell IM. (2010). Conventional myosins—unconventional functions. Biophys Rev , 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Roberts R, Pohl M, Nigam S, Kreidberg J, Wang Z, Heino J, Ivaska J, Coffa S, Harris RC, et al. (2004). Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am J Physiol Renal Physiol , F602–F611. [DOI] [PubMed] [Google Scholar]
- Cheng F, Miao L, Wu Q, Gong X, Xiong J, Zhang J. (2016). Vinculin b deficiency causes epicardial hyperplasia and coronary vessel disorganization in zebrafish. Development , 3522–3531. [DOI] [PubMed] [Google Scholar]
- Chlasta J, Milani P, Runel G, Duteyrat JL, Arias L, Lamire LA, Boudaoud A, Grammont M. (2017). Variations in basement membrane mechanics are linked to epithelial morphogenesis. Development , 4350–4362. [DOI] [PubMed] [Google Scholar]
- Chouhan BS, Kapyla J, Denessiouk K, Denesyuk A, Heino J, Johnson MS. (2014). Early chordate origin of the vertebrate integrin alphaI domains. PLoS One , e112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulos A, Thorpe SD, Cortes E, Lachowski D, Rice AJ, Mykuliak VV, Rog T, Lee DA, Hytonen VP, Del Rio Hernandez AE. (2020). Syndecan-4 tunes cell mechanics by activating the kindlin-integrin-RhoA pathway. Nat Mater , 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuba K, Hawkes W, Tojkander S, Kogan K, Engel U, Iskratsch T, Lappalainen P. (2018). Calponin-3 is critical for coordinated contractility of actin stress fibers. Sci Rep , 17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Dor Y. (2012). Vascular instruction of pancreas development. Development , 2833–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S, Strandkvist C, Bathmann J, de Gennes M, Kabla A, Salbreux G, Baum B. (2017). Myosin II controls junction fluctuations to guide epithelial tissue ordering. Dev Cell , 480–492.e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. (1994). Integrin switching regulates normal trophoblast invasion. Development , 3657–3666. [DOI] [PubMed] [Google Scholar]
- Daniel E, Cleaver O. (2019). Vascularizing organogenesis: lessons from developmental biology and implications for regenerative medicine. Curr Top Dev Biol , 177–220. [DOI] [PubMed] [Google Scholar]
- Dasbiswas K, Hu S, Schnorrer F, Safran SA, Bershadsky AD. (2018). Ordering of myosin II filaments driven by mechanical forces: experiments and theory. Philos Trans R Soc Lond B Biol Sci , 20170114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Dzamba BD, Keller R, Desimone DW. (2008). Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev Dyn , 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Hoffstrom BG, Keller R, DeSimone DW. (2002). Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha(5)beta(1), fibronectin, and tissue geometry. Dev Biol , 109–129. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Keller R, DeSimone DW. (2004). Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev Dyn , 888–895. [DOI] [PubMed] [Google Scholar]
- Dehal P, Boore JL. (2005). Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol , e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon I, Brown NH. (2009). The integrin adhesion complex changes its composition and function during morphogenesis of an epithelium. J Cell Sci , 4363–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Sun X, Bui GA. (2014). Force transmission at cell-cell and cell-matrix adhesions. Biochemistry , 7706–7717. [DOI] [PubMed] [Google Scholar]
- Diogo R, Kelly RG, Christiaen L, Levine M, Ziermann JM, Molnar JL, Noden DM, Tzahor E. (2015). A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature , 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Gimenez P, Brown NH, Martin-Bermudo MD. (2007). Integrin-ECM interactions regulate the changes in cell shape driving the morphogenesis of the Drosophila wing epithelium. J Cell Sci , 1061–1071. [DOI] [PubMed] [Google Scholar]
- Doyle AD, Kutys ML, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. (2012). Micro-environmental control of cell migration — myosin IIA is required for efficient migration in fibrillar environments through control of cell adhesion dynamics. J Cell Sci , 2244–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper GW, Shoemark DK, Adams JC. (2019). Modelling the early evolution of extracellular matrix from modern ctenophores and sponges. Essays Biochem , 389–405. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Wagner J. (1986). Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. J Cell Biol , 1738–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband JL, Thiery JP. (1987). Distribution of laminin and collagens during avian neural crest development. Development , 461–478. [DOI] [PubMed] [Google Scholar]
- Eder D, Basler K, Aegerter CM. (2017). Challenging FRET-based E-cadherin force measurements in Drosophila. Sci Rep , 13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Faitelson L, Ravid S. (2006). PAK1 and aPKCzeta regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol Biol Cell , 2869–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan R, Huxley-Jones J, Mould AP, Humphries MJ, Robertson DL, Boot-Handford RP. (2005). The integrins of the urochordate Ciona intestinalis provide novel insights into the molecular evolution of the vertebrate integrin family. BMC Evol Biol , 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito JY, Valcourt U, Cluzel C, Lethias C. (2010). The fibrillar collagen family. Int J Mol Sci , 407–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Wu J-J. (2005). Collagen cross-links. Top Curr Chem , 207–229. [Google Scholar]
- Fahey B, Degnan BM. (2012). Origin and evolution of laminin gene family diversity. Mol Biol Evol , 1823–1836. [DOI] [PubMed] [Google Scholar]
- Fath KR, Edgell CJ, Burridge K. (1989). The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Sci (Pt 1), 67–75. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA. (2009). Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell , 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Zallen JA. (2011). Oscillatory behaviors and hierarchical assembly of contractile structures in intercalating cells. Phys Biol , 045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Zallen JA. (2013). Wounded cells drive rapid epidermal repair in the early Drosophila embryo. Mol Biol Cell , 3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler AL, Boudko SP, Rokas A, Hudson BG. (2018). The triple helix of collagens—an ancient protein structure that enabled animal multicellularity and tissue evolution. J Cell Sci , jcs203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler AL, Darris CE, Chetyrkin SV, Pedchenko VK, Boudko SP, Brown KL, Gray Jerome W, Hudson JK, Rokas A, Hudson BG. (2017). Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. eLife , e24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla MS, Dimeo KD, Tong T, Peters DM. (2017). Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp Eye Res , 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foolen J, Janssen-van den Broek MW, Baaijens FP. (2014). Synergy between Rho signaling and matrix density in cyclic stretch-induced stress fiber organization. Acta Biomater , 1876–1885. [DOI] [PubMed] [Google Scholar]
- Gehler S, Baldassarre M, Lad Y, Leight JL, Wozniak MA, Riching KM, Eliceiri KW, Weaver VM, Calderwood DA, Keely PJ. (2009). Filamin A-beta1 integrin complex tunes epithelial cell response to matrix tension. Mol Biol Cell , 3224–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George L, Dunkel H, Hunnicutt BJ, Filla M, Little C, Lansford R, Lefcort F. (2016). In vivo time-lapse imaging reveals extensive neural crest and endothelial cell interactions during neural crest migration and formation of the dorsal root and sympathetic ganglia. Dev Biol , 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RH. (1991). Control of type I collagen formation in the lung. Am J Physiol , L29–L40. [DOI] [PubMed] [Google Scholar]
- Goodwin K, Ellis SJ, Lostchuck E, Zulueta-Coarasa T, Fernandez-Gonzalez R, Tanentzapf G. (2016). Basal cell-extracellular matrix adhesion regulates force transmission during tissue morphogenesis. Dev Cell , 611–625. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Wagner GP. (2017). The placenta as a model for understanding the origin and evolution of vertebrate organs. Nat Ecol Evol , 72. [DOI] [PubMed] [Google Scholar]
- Grossman M, Ben-Chetrit N, Zhuravlev A, Afik R, Bassat E, Solomonov I, Yarden Y, Sagi I. (2016). Tumor cell invasion can be blocked by modulators of collagen fibril alignment that control assembly of the extracellular matrix. Cancer Res , 4249–4258. [DOI] [PubMed] [Google Scholar]
- Gutzman JH, Sahu SU, Kwas C. (2015). Non-muscle myosin IIA and IIB differentially regulate cell shape changes during zebrafish brain morphogenesis. Dev Biol , 103–115. [DOI] [PubMed] [Google Scholar]
- Hall MS, Alisafaei F, Ban E, Feng X, Hui CY, Shenoy VB, Wu M. (2016). Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc Natl Acad Sci USA , 14043–14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Chen S, Yuan W, Fan Q, Tian J, Wang X, Chen L, Zhang X, Wei W, Liu R, et al. (2016). Oriented collagen fibers direct tumor cell intravasation. Proc Natl Acad Sci USA , 11208–11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YL, Ronceray P, Xu G, Malandrino A, Kamm RD, Lenz M, Broedersz CP, Guo M. (2018). Cell contraction induces long-ranged stress stiffening in the extracellular matrix. Proc Natl Acad Sci USA , 4075–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatte G, Prigent C, Tassan JP. (2018). Tight junctions negatively regulate mechanical forces applied to adherens junctions in vertebrate epithelial tissue. J Cell Sci , jcs208736. [DOI] [PubMed] [Google Scholar]
- Heissler SM, Sellers JR. (2016). Various themes of myosin regulation. J Mol Biol , 1927–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuze ML, Sankara Narayana GHN, D’Alessandro J, Cellerin V, Dang T, Williams DS, Van Hest JC, Marcq P, Mege RM, Ladoux B. (2019). Myosin II isoforms play distinct roles in adherens junction biogenesis. eLife , e46599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, Langenbach KJ. (2000). Stimulation of integrin-mediated cell contractility by fibronectin polymerization. J Biol Chem , 10673–10682. [DOI] [PubMed] [Google Scholar]
- Hoffmann FG, Opazo JC, Storz JF. (2012). Whole-genome duplications spurred the functional diversification of the globin gene superfamily in vertebrates. Mol Biol Evol , 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland LZ, Ocampo Daza D. (2018). A new look at an old question: when did the second whole genome duplication occur in vertebrate evolution? Genome Biol , 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Cui D, Yang X, Hu J, Wan W, Zeng J. (2011). The crucial role of collagen-binding integrins in maintaining the mechanical properties of human scleral fibroblasts-seeded collagen matrix. Mol Vis , 1334–1342. [PMC free article] [PubMed] [Google Scholar]
- Hu S, Dasbiswas K, Guo Z, Tee YH, Thiagarajan V, Hersen P, Chew TL, Safran SA, Zaidel-Bar R, Bershadsky AD. (2017). Long-range self-organization of cytoskeletal myosin II filament stacks. Nat Cell Biol , 133–141. [DOI] [PubMed] [Google Scholar]
- Hufton AL, Groth D, Vingron M, Lehrach H, Poustka AJ, Panopoulou G. (2008). Early vertebrate whole genome duplications were predated by a period of intense genome rearrangement. Genome Res , 1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtala M, Heino J, Casciari D, de Luise A, Johnson MS. (2005). Integrin evolution: insights from ascidian and teleost fish genomes. Matrix Biol , 83–95. [DOI] [PubMed] [Google Scholar]
- Huxley-Jones J, Robertson DL, Boot-Handford RP. (2007). On the origins of the extracellular matrix in vertebrates. Matrix Biol , 2–11. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Kita M, Matsui TS, Nagasato AI, Araki T, Chiang SH, Sezaki T, Kimura Y, Ueda K, Deguchi S, et al. (2017). Vinexin family (SORBS) proteins play different roles in stiffness-sensing and contractile force generation. J Cell Sci , 3517–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KD, Wieschaus E. (1994). Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development , 827–841. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Fletcher DA, Reinhart-King CA. (2020). Stiffness sensing by cells. Physiol Rev , 695–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Sun Z, Chen X, Li J, Xu Y, Zu Y, Hu J, Han D, Yang C. (2016). Cells sensing mechanical cues: stiffness influences the lifetime of cell-extracellular matrix interactions by affecting the loading rate. ACS Nano , 207–217. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Chouhan BS. (2014). Evolution of integrin I domains. Adv Exp Med Biol , 1–19. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Dadu E, Nykvist P, Kapyla J, White DJ, Ivaska J, Vehvilainen P, Reunanen H, Larjava H, Hakkinen L, Heino J. (2004). Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem , 31956–31963. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Baldock C, Bella J, Boot-Handford RP. (2007). Collagens at a glance. J Cell Sci , 1955–1958. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird EG. (2008). Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol , 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunas R, Nguyen P, Usami S, Chien S. (2005). Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci USA , 15895–15900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K, Suzuki T, Weiss KM. (2004). Genetic basis for the evolution of vertebrate mineralized tissue. Proc Natl Acad Sci USA , 11356–11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya M, Higuchi H. (2010). Nonlinear elasticity and an 8-nm working stroke of single myosin molecules in myofilaments. Science , 686–689. [DOI] [PubMed] [Google Scholar]
- Keller R, Shih J, Domingo C. (1992). The patterning and functioning of protrusive activity during convergence and extension of the Xenopus organiser. Dev Suppl 81–91. [PubMed] [Google Scholar]
- Keller R, Winklbauer R. (1992). Cellular basis of amphibian gastrulation. Curr Top Dev Biol , 39–89. [DOI] [PubMed] [Google Scholar]
- Keller RE. (1986). The cellular basis of amphibian gastrulation. Dev Biol (NY 1985) , 241–327. [DOI] [PubMed] [Google Scholar]
- Kick K, Nekolla K, Rehberg M, Vollmar AM, Zahler S. (2016). New view on endothelial cell migration: switching modes of migration based on matrix composition. Arterioscler Thromb Vasc Biol , 2346–2357. [DOI] [PubMed] [Google Scholar]
- Kim HT, Yin W, Jin YJ, Panza P, Gunawan F, Grohmann B, Buettner C, Sokol AM, Preussner J, Guenther S, et al. (2018). Myh10 deficiency leads to defective extracellular matrix remodeling and pulmonary disease. Nat Commun , 4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V. (2009). Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci USA , 18267–18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn JC, Lampi MC, Reinhart-King CA. (2015). Age-related vascular stiffening: causes and consequences. Front Genet , 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. (2009). Demonstration of catch bonds between an integrin and its ligand. J Cell Biol , 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D, Luna S, Vogel V. (2015). Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun , 8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuragano M, Murakami Y, Takahashi M. (2018a). Nonmuscle myosin IIA and IIB differentially contribute to intrinsic and directed migration of human embryonic lung fibroblasts. Biochem Biophys Res Commun , 25–31. [DOI] [PubMed] [Google Scholar]
- Kuragano M, Uyeda TQP, Kamijo K, Murakami Y, Takahashi M. (2018b). Different contributions of nonmuscle myosin IIA and IIB to the organization of stress fiber subtypes in fibroblasts. Mol Biol Cell , 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisu S, Takenawa T. (2009). The WASP and WAVE family proteins. Genome Biol , 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Ueda K, Kioka N. (2018). Vinexin family (SORBS) proteins regulate mechanotransduction in mesenchymal stem cells. Sci Rep , 11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwansa AL, De Vita R, Freeman JW. (2016). Tensile mechanical properties of collagen type I and its enzymatic crosslinks. Biophys Chem , 1–10. [DOI] [PubMed] [Google Scholar]
- Lagendijk AK, Gomez GA, Baek S, Hesselson D, Hughes WE, Paterson S, Conway DE, Belting HG, Affolter M, Smith KA, et al. (2017). Live imaging molecular changes in junctional tension upon VE-cadherin in zebrafish. Nat Commun , 1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. (2003). Role of endothelial cells in early pancreas and liver development. Mech Dev , 59–64. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Silver FH. (2009). Mineral deposition in the extracellular matrices of vertebrate tissues: identification of possible apatite nucleation sites on type I collagen. Cells Tissues Organs , 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin M, Gomez NC, Levorse J, Sendoel A, Sribour M, Fuchs E. (2019). An RNAi screen unravels the complexities of Rho GTPase networks in skin morphogenesis. eLife , e50226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausecker F, Tian X, Inoue K, Wang Z, Pedigo CE, Hassan H, Liu C, Zimmer M, Jinno S, Huckle AL, et al. (2018). Vinculin is required to maintain glomerular barrier integrity. Kidney Int , 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S, Hu X, Yao M, Chen H, Yu M, Xu X, Nakazawa N, Margadant FM, Sheetz MP, Yan J. (2017). Mechanotransmission and mechanosensing of human alpha-actinin 1. Cell Rep , 2714–2723. [DOI] [PubMed] [Google Scholar]
- Le S, Yu M, Yan J. (2019). Direct single-molecule quantification reveals unexpectedly high mechanical stability of vinculin-talin/alpha-catenin linkages. Sci Adv , eaav2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon CA, Weinberg SH. (2017). Multiple cryptic binding sites are necessary for robust fibronectin assembly: an in silico study. Sci Rep , 18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne A, Geiger B. (2016). The inner workings of stress fibers—from contractile machinery to focal adhesions and back. J Cell Sci , 1293–1304. [DOI] [PubMed] [Google Scholar]
- Lohner J, Rupprecht J-F, U. Hu, Mandriota N, Saxena M, de Araujo DP, Hone J, Sahin O, Prost J, Sheetz MP. (2019). Large and reversible myosin-dependent forces in rigidity sensing. Nat Phys , 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis H, Kakou A, Regnault V, Labat C, Bressenot A, Gao-Li J, Gardner H, Thornton SN, Challande P, Li Z, Lacolley P. (2007). Role of alpha1beta1-integrin in arterial stiffness and angiotensin-induced arterial wall hypertrophy in mice. Am J Physiol Heart Circ Physiol , H2597–H2604. [DOI] [PubMed] [Google Scholar]
- Maartens AP, Wellmann J, Wictome E, Klapholz B, Green H, Brown NH. (2016). Drosophila vinculin is more harmful when hyperactive than absent, and can circumvent integrin to form adhesion complexes. J Cell Sci , 4354–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marg S, Winkler U, Sestu M, Himmel M, Schonherr M, Bar J, Mann A, Moser M, Mierke CT, Rottner K, et al. (2010). The vinculin-DeltaIn20/21 mouse: characteristics of a constitutive, actin-binding deficient splice variant of vinculin. PLoS One , e11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani RA, Paranjpe S, Dobrowolski R, Weber GF. (2020). 14–3-3 targets keratin intermediate filaments to mechanically sensitive cell-cell contacts. Mol Biol Cell , 930–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletaz F, Firbas PN, Maeso I, Tena JJ, Bogdanovic O, Perry M, Wyatt CDR, de la Calle-Mustienes E, Bertrand S, Burguera D, et al. (2018). Amphioxus functional genomics and the origins of vertebrate gene regulation. Nature , 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. (2003). Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr Biol , 1182–1191. [DOI] [PubMed] [Google Scholar]
- Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. (2010). Integration of contractile forces during tissue invagination. J Cell Biol , 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. (2011). Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci USA , 4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacci G, Wolfenson H, Liu S, Stachowiak MR, Iskratsch T, Mathur A, Ghassemi S, Gauthier N, Tabdanov E, Lohner J, et al. (2016). alpha-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol Biol Cell , 3471–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli L, Billington N, Sun SA, Bird JE, Nagy A, Friedman TB, Takagi Y, Sellers JR. (2018). Bipolar filaments of human nonmuscle myosin 2-A and 2-B have distinct motile and mechanical properties. eLife , e32871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzenga R, Mitsi M. (2019). The molecular dance of fibronectin: conformational flexibility leads to functional versatility. Biomacromolecules , 55–72. [DOI] [PubMed] [Google Scholar]
- Miron-Mendoza M, Seemann J, Grinnell F. (2008). Collagen fibril flow and tissue translocation coupled to fibroblast migration in 3D collagen matrices. Mol Biol Cell , 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron-Mendoza M, Seemann J, Grinnell F. (2010). The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials , 6425–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi H, Arora PD, Simmons CA, Janmey PA, McCulloch CA. (2015a). Inelastic behaviour of collagen networks in cell-matrix interactions and mechanosensation. J R Soc Interface , 20141074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi H, Pinto VI, Wang Y, Hinz B, Janmey PA, McCulloch CA. (2015b). Filamin A mediates wound closure by promoting elastic deformation and maintenance of tension in the collagen matrix. J Invest Dermatol , 2852–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan-Earley R, Dvorak AM, Aird WC. (2013). Evolutionary origins of the blood vascular system and endothelium. J Thromb Haemost (Suppl 1), 46–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Chapuli R, Carmona R, Guadix JA, Macias D, Perez-Pomares JM. (2005). The origin of the endothelial cells: an evo-devo approach for the invertebrate/vertebrate transition of the circulatory system. Evol Dev , 351–358. [DOI] [PubMed] [Google Scholar]
- Murakami N, Chauhan VP, Elzinga M. (1998). Two nonmuscle myosin II heavy chain isoforms expressed in rabbit brains: filament forming properties, the effects of phosphorylation by protein kinase C and casein kinase II, and location of the phosphorylation sites. Biochemistry , 1989–2003. [DOI] [PubMed] [Google Scholar]
- Murakami N, Kotula L, Hwang YW. (2000). Two distinct mechanisms for regulation of nonmuscle myosin assembly via the heavy chain: phosphorylation for MIIB and mts 1 binding for MIIA. Biochemistry , 11441–11451. [DOI] [PubMed] [Google Scholar]
- Nagy N, Mwizerwa O, Yaniv K, Carmel L, Pieretti-Vanmarcke R, Weinstein BM, Goldstein AM. (2009). Endothelial cells promote migration and proliferation of enteric neural crest cells via beta1 integrin signaling. Dev Biol , 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y, Takeda H, Kohara Y, Morishita S. (2007). Reconstruction of the vertebrate ancestral genome reveals dynamic genome reorganization in early vertebrates. Genome Res , 1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. (1991). Demonstration of actin filament stress fibers in microvascular endothelial cells in situ. Microvasc Res , 103–112. [DOI] [PubMed] [Google Scholar]
- Niland S, Westerhausen C, Schneider SW, Eckes B, Schneider MF, Eble JA. (2011). Biofunctionalization of a generic collagenous triple helix with the alpha2beta1 integrin binding site allows molecular force measurements. Int J Biochem Cell Biol , 721–731. [DOI] [PubMed] [Google Scholar]
- Nuhn JAM, Perez AM, Schneider IC. (2018). Contact guidance diversity in rotationally aligned collagen matrices. Acta Biomater , 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes PW, Beckham Y, Stricker J, Gardel ML. (2012). Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J Cell Biol , 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brown NM, Pfau SJ, Gu C. (2018). Bridging barriers: a comparative look at the blood-brain barrier across organisms. Genes Dev , 466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Wen FL, Sami MM, Shibata T, Hayashi S. (2018). A switch-like activation relay of EGFR-ERK signaling regulates a wave of cellular contractility for epithelial invagination. Dev Cell , 162–172.e165. [DOI] [PubMed] [Google Scholar]
- Park HJ, Park JE, Lee H, Kim SJ, Yun JI, Kim M, Park KH, Lee ST. (2017). Integrins functioning in uterine endometrial stromal and epithelial cells in estrus. Reproduction , 351–360. [DOI] [PubMed] [Google Scholar]
- Pathan-Chhatbar S, Taft MH, Reindl T, Hundt N, Latham SL, Manstein DJ. (2018). Three mammalian tropomyosin isoforms have different regulatory effects on nonmuscle myosin-2B and filamentous beta-actin in vitro. J Biol Chem , 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. (2007). Actin stress fibres. J Cell Sci , 3491–3499. [DOI] [PubMed] [Google Scholar]
- Perris R, Krotoski D, Bronner-Fraser M. (1991). Collagens in avian neural crest development: distribution in vivo and migration-promoting ability in vitro. Development , 969–984. [DOI] [PubMed] [Google Scholar]
- Pichard V, Honore S, Kovacic H, Li C, Prevot C, Briand C, Rognoni JB. (2001). Adhesion, actin cytoskeleton organisation and the spreading of colon adenocarcinoma cells induced by EGF are mediated by alpha2beta1 integrin low clustering through focal adhesion kinase. Histochem Cell Biol , 337–348. [DOI] [PubMed] [Google Scholar]
- Pins GD, Christiansen DL, Patel R, Silver FH. (1997). Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J , 2164–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. (2012). Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell , 1513–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov C, Radic T, Haasters F, Prall WC, Aszodi A, Gullberg D, Schieker M, Docheva D. (2011). Integrins alpha2beta1 and alpha11beta1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis , e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post A, Wang E, Cosgriff-Hernandez E. (2019). A review of integrin-mediated endothelial cell phenotype in the design of cardiovascular devices. Ann Biomed Eng , 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleo JI, Parker SS, Roman MR, Watson AW, Eliato KR, Peng L, Saboda K, Roe DJ, Ros R, Gertler FB, Mouneimne G. (2019). Mechanosensing during directed cell migration requires dynamic actin polymerization at focal adhesions. J Cell Biol , 4215–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Park BO, Liu J, Chen B, Choesmel-Cadamuro V, Belguise K, Heo WD, Wang X. (2017). Cell-matrix adhesion and cell-cell adhesion differentially control basal myosin oscillation and Drosophila egg chamber elongation. Nat Commun , 14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Butler JM, Ding BS. (2016). Angiocrine functions of organ-specific endothelial cells. Nature , 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimzadeh J, Meng F, Sachs F, Wang J, Verma D, Hua SZ. (2011). Real-time observation of flow-induced cytoskeletal stress in living cells. Am J Physiol Cell Physiol , C646–C652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy SK, Kusumbe AP, Adams RH. (2015). Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol , 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JW, DeSimone DW. (1996). Xenopus embryonic cell adhesion to fibronectin: position-specific activation of RGD/synergy site-dependent migratory behavior at gastrulation. J Cell Biol , 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SA, D’Ambrosio MV, Vale RD. (2014). Induction of focal adhesions and motility in Drosophila S2 cells. Mol Biol Cell , 3861–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard-Blum S. (2011). The collagen family. Cold Spring Harb Perspect Biol , a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge LA, Mitchell K, Al-Anbaki A, Shaikh Qureshi WM, Stephen LA, Tenin G, Lu Y, Lupu IE, Clowes C, Robertson A, et al. (2017). Non-muscle myosin IIB (Myh10) is required for epicardial function and coronary vessel formation during mammalian development. PLoS Genet , e1007068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riopel M, Wang R. (2014). Collagen matrix support of pancreatic islet survival and function. Front Biosci (Landmark Ed) , 77–90. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. (2013). Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc Natl Acad Sci USA , E1361–E1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. (2009). Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc Natl Acad Sci USA , 16245–16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P, Iskratsch T, Sheetz MP. (2012). Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci , 3025–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan W, McMeeking RM, Chen CS, McGarry JP, Deshpande VS. (2015). Cooperative contractility: the role of stress fibres in the regulation of cell-cell junctions. J Biomech , 520–528. [DOI] [PubMed] [Google Scholar]
- Rozario T, DeSimone DW. (2010). The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol , 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelman EU, Keely PJ, Santoro SA. (1995). Loss of MDCK cell alpha 2 beta 1 integrin expression results in reduced cyst formation, failure of hepatocyte growth factor/scatter factor-induced branching morphogenesis, and increased apoptosis. J Cell Sci (Pt 11), 3531–3540. [DOI] [PubMed] [Google Scholar]
- San Antonio JD, Zoeller JJ, Habursky K, Turner K, Pimtong W, Burrows M, Choi S, Basra S, Bennett JS, DeGrado WF, Iozzo RV. (2009). A key role for the integrin alpha2beta1 in experimental and developmental angiogenesis. Am J Pathol , 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho A, Vandersmissen I, Craps S, Luttun A, Groll J. (2017). A new strategy to measure intercellular adhesion forces in mature cell-cell contacts. Sci Rep , 46152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandquist JC, Means AR. (2008). The C-terminal tail region of nonmuscle myosin II directs isoform-specific distribution in migrating cells. Mol Biol Cell , 5156–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghvi-Shah R, Weber GF. (2017). Intermediate filaments at the junction of mechanotransduction, migration, and development. Front Cell Dev Biol , 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sao K, Jones TM, Doyle AD, Maity D, Schevzov G, Chen Y, Gunning PW, Petrie RJ. (2019). Myosin II governs intracellular pressure and traction by distinct tropomyosin-dependent mechanisms. Mol Biol Cell , 1170–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker B, Bagchi A, Walter C, Almeida J, Pathak A. (2019). Longer collagen fibers trigger multicellular streaming on soft substrates via enhanced forces and cell-cell cooperation. J Cell Sci , jcs226753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer JK, Choi W, Jung KC, He L, Harris NJ, Peifer M. (2011). A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension. Mol Biol Cell , 2491–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffhauer ES, Luo T, Mohan K, Srivastava V, Qian X, Griffis ER, Iglesias PA, Robinson DN. (2016). Mechanoaccumulative elements of the mammalian actin cytoskeleton. Curr Biol , 1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte K, Kunter U, Moeller MJ. (2015). The evolution of blood pressure and the rise of mankind. Nephrol Dial Transplant , 713–723. [DOI] [PubMed] [Google Scholar]
- Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. (1997). Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci USA , 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZL, Dodge MR, Kahn H, Ballarini R, Eppell SJ. (2008). Stress-strain experiments on individual collagen fibrils. Biophys J , 3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZL, Kahn H, Ballarini R, Eppell SJ. (2011). Viscoelastic properties of isolated collagen fibrils. Biophys J , 3008–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett LA, Tiede-Lewis LM, Xie Y, Lu Y, Ray EC, Dallas SL. (2019). Collagen dynamics during the process of osteocyte embedding and mineralization. Front Cell Dev Biol , 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigei T, Tsuru H, Ishikawa N, Yoshioka K. (2001). Absence of endothelium in invertebrate blood vessels: significance of endothelium and sympathetic nerve/medial smooth muscle in the vertebrate vascular system. Jpn J Pharmacol , 253–260. [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R. (1992). Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development , 901–914. [DOI] [PubMed] [Google Scholar]
- Shimeld SM, Holland PW. (2000). Vertebrate innovations. Proc Natl Acad Sci USA , 4449–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo A. (2018). Models of convergent extension during morphogenesis. Wiley Interdiscip Rev Dev Biol , e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook DR, Kasprowicz EM, Davidson LA, Keller R. (2018). Large, long range tensile forces drive convergence during Xenopus blastopore closure and body axis elongation. eLife , e26944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver FH. (2009). The importance of collagen fibers in vertebrate biology. J Eng Fiber Fabr , 9–17. [Google Scholar]
- Silver FH, Ebrahimi A, Snowhill PB. (2002). Viscoelastic properties of self-assembled type I collagen fibers: molecular basis of elastic and viscous behaviors. Connect Tissue Res , 569–580. [PubMed] [Google Scholar]
- Silver FH, Horvath I, Foran DJ. (2001). Viscoelasticity of the vessel wall: the role of collagen and elastic fibers. Crit Rev Biomed Eng , 279–301. [DOI] [PubMed] [Google Scholar]
- Sim JY, Moeller J, Hart KC, Ramallo D, Vogel V, Dunn AR, Nelson WJ, Pruitt BL. (2015). Spatial distribution of cell-cell and cell-ECM adhesions regulates force balance while maintaining E-cadherin molecular tension in cell pairs. Mol Biol Cell , 2456–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Carraher C, Schwarzbauer JE. (2010). Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol , 397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. (2010). Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol , 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinet S, Vitale ML. (2008). Isoform B of myosin II heavy chain mediates actomyosin contractility during TNFalpha-induced apoptosis. J Cell Sci , 1681–1692. [DOI] [PubMed] [Google Scholar]
- Soslau G. (2020). The role of the red blood cell and platelet in the evolution of mammalian and avian endothermy. J Exp Zool B Mol Dev Evol , 113–127. [DOI] [PubMed] [Google Scholar]
- Stam S, Alberts J, Gardel ML, Munro E. (2015). Isoforms confer characteristic force generation and mechanosensation by myosin II filaments. Biophys J , 1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens CH, Orr KS, Acton AJ, Tersey SA, Mirmira RG, Considine RV, Voytik-Harbin SL. (2018). In situ type I oligomeric collagen macroencapsulation promotes islet longevity and function in vitro and in vivo. Am J Physiol Endocrinol Metab , E650–E661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson A, Adams JW, Vaccarezza M. (2017). The vertebrate heart: an evolutionary perspective. J Anat , 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker J, Beckham Y, Davidson MW, Gardel ML. (2013). Myosin II-mediated focal adhesion maturation is tension insensitive. PLoS One , e70652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson RB, Hassenkam T, Grant CA, Magnusson SP. (2010). Tensile properties of human collagen fibrils and fascicles are insensitive to environmental salts. Biophys J , 4020–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney SM, DiLullo G, Slater SJ, Martinez J, Iozzo RV, Lauer-Fields JL, Fields GB, San Antonio JD. (2003). Angiogenesis in collagen I requires alpha2beta1 ligation of a GFP*GER sequence and possibly p38 MAPK activation and focal adhesion disassembly. J Biol Chem , 30516–30524. [DOI] [PubMed] [Google Scholar]
- Sztal T, Berger S, Currie PD, Hall TE. (2011). Characterization of the laminin gene family and evolution in zebrafish. Dev Dyn , 422–431. [DOI] [PubMed] [Google Scholar]
- Tamariz E, Grinnell F. (2002). Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell , 3915–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee SY, Fu J, Chen CS, Janmey PA. (2011). Cell shape and substrate rigidity both regulate cell stiffness. Biophys J , L25–L27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teravainen TP, Myllymaki SM, Friedrichs J, Strohmeyer N, Moyano JV, Wu C, Matlin KS, Muller DJ, Manninen A. (2013). alphaV-integrins are required for mechanotransduction in MDCK epithelial cells. PLoS One , e71485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiger CF, Fougerousse F, Grundstrom G, Velling T, Gullberg D. (2001). alpha11beta1 Integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev Biol , 116–129. [DOI] [PubMed] [Google Scholar]
- Tojkander S, Gateva G, Lappalainen P. (2012). Actin stress fibers—assembly, dynamics and biological roles. J Cell Sci , 1855–1864. [DOI] [PubMed] [Google Scholar]
- Tondon A, Kaunas R. (2014). The direction of stretch-induced cell and stress fiber orientation depends on collagen matrix stress. PLoS One , e89592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichet L, Le Digabel J, Hawkins RJ, Vedula SR, Gupta M, Ribrault C, Hersen P, Voituriez R, Ladoux B. (2012). Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc Natl Acad Sci USA , 6933–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta D, Jones JC. (2003). The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci , 4977–4984. [DOI] [PubMed] [Google Scholar]
- Tuckwell D, Calderwood DA, Green LJ, Humphries MJ. (1995). Integrin alpha 2 I-domain is a binding site for collagens. J Cell Sci (Pt 4), 1629–1637. [DOI] [PubMed] [Google Scholar]
- Tulla M, Pentikainen OT, Viitasalo T, Kapyla J, Impola U, Nykvist P, Nissinen L, Johnson MS, Heino J. (2001). Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 10I domains. J Biol Chem , 48206–48212. [DOI] [PubMed] [Google Scholar]
- Turner KR, Adams C, Staelens S, Deckmyn H, San Antonio J. (2020). Crucial role for endothelial cell alpha2beta1 integrin receptor clustering in collagen-induced angiogenesis. Anat Rec (Hoboken) , 1604–1618. [DOI] [PubMed] [Google Scholar]
- Ushiki T. (2002). Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol , 109–126. [DOI] [PubMed] [Google Scholar]
- Vandepoele K, De Vos W, Taylor JS, Meyer A, Van de Peer Y. (2004). Major events in the genome evolution of vertebrates: paranome age and size differ considerably between ray-finned fishes and land vertebrates. Proc Natl Acad Sci USA , 1638–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Rijt JA, van der Werf KO, Bennink ML, Dijkstra PJ, Feijen J. (2006). Micromechanical testing of individual collagen fibrils. Macromol Biosci , 697–702. [DOI] [PubMed] [Google Scholar]
- van Geemen D, Smeets MW, van Stalborch AM, Woerdeman LA, Daemen MJ, Hordijk PL, Huveneers S. (2014). F-actin-anchored focal adhesions distinguish endothelial phenotypes of human arteries and veins. Arterioscler Thromb Vasc Biol , 2059–2067. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. (2007). Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol , 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vize PD, Smith HW. (2004). A Homeric view of kidney evolution: a reprint of H.W. Smith’s classic essay with a new introduction. Evolution of the kidney (1943). Anat Rec A Discov Mol Cell Evol Biol , 344–354. [DOI] [PubMed] [Google Scholar]
- Walcott S, Sun SX. (2010). A mechanical model of actin stress fiber formation and substrate elasticity sensing in adherent cells. Proc Natl Acad Sci USA , 7757–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Abhilash AS, Chen CS, Wells RG, Shenoy VB. (2014). Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys J , 2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan MC, Senger DR. (2003). Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem , 327–334. [DOI] [PubMed] [Google Scholar]
- Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. (2009). Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol , 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. (2003). Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood , 3262–3269. [DOI] [PubMed] [Google Scholar]
- Wolfenson H, Bershadsky A, Henis YI, Geiger B. (2011). Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J Cell Sci , 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S, Guo WH, Wang YL. (2014). Fibroblasts probe substrate rigidity with filopodia extensions before occupying an area. Proc Natl Acad Sci USA , 17176–17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Bao M, Bruekers SMC, Huck WTS. (2017). Collagen gels with different fibrillar microarchitectures elicit different cellular responses. ACS Appl Mater Interfaces , 19630–19637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Gilbert M, Petley-Ragan L, Auld VJ. (2014). Loss of focal adhesions in glia disrupts both glial and photoreceptor axon migration in the Drosophila visual system. Development , 3072–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Baribault H, Adamson ED. (1998). Vinculin knockout results in heart and brain defects during embryonic development. Development , 327–337. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gurusiddappa S, Rich RL, Owens RT, Keene DR, Mayne R, Hook A, Hook M. (2000). Multiple binding sites in collagen type I for the integrins alpha1beta1 and alpha2beta1. J Biol Chem , 38981–38989. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Tsuboi T, Ishinabe N, Kitaguchi T, Michiue T. (2016). Wide and high resolution tension measurement using FRET in embryo. Sci Rep , 28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, van der Werf KO, Fitie CF, Bennink ML, Dijkstra PJ, Feijen J. (2008). Mechanical properties of native and cross-linked type I collagen fibrils. Biophys J , 2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye N, Verma D, Meng F, Davidson MW, Suffoletto K, Hua SZ. (2014). Direct observation of alpha-actinin tension and recruitment at focal adhesions during contact growth. Exp Cell Res , 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylanne J, Chen Y, O’Toole TE, Loftus JC, Takada Y, Ginsberg MH. (1993). Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol , 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Jiang H, Kim M, Vink J, Cremers S, Paik D, Wapner R, Mahendroo M, Myers K. (2014). Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PLoS One , e112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. (2004). Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell , 343–355. [DOI] [PubMed] [Google Scholar]
- Zambuto SG, Clancy KBH, Harley BAC. (2019). A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus , 20190016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltz C, Gullberg D. (2016). The integrin-collagen connection—a glue for tissue repair? J Cell Sci , 1284. [DOI] [PubMed] [Google Scholar]
- Zemljic-Harpf AE, Ponrartana S, Avalos RT, Jordan MC, Roos KP, Dalton ND, Phan VQ, Adamson ED, Ross RS. (2004). Heterozygous inactivation of the vinculin gene predisposes to stress-induced cardiomyopathy. Am J Pathol , 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Bothe I, Hirche F, Zweers M, Gullberg D, Pfitzer G, Krieg T, Eckes B, Aumailley M. (2006). Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins: contribution of alpha2beta1 integrin. J Cell Sci , 1886–1895. [DOI] [PubMed] [Google Scholar]
- Zhou DW, Lee TT, Weng S, Fu J, Garcia AJ. (2017). Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions. Mol Biol Cell , 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger AJ, Xu H, Figueiredo J, Paredes J, Seruca R, Stamenovic D, Smith ML. (2018). Dependence of tensional homeostasis on cell type and on cell-cell interactions. Cell Mol Bioeng , 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]