Abstract
Objective:
To examine significance of sarcoma dominance (SD) patterns In uterine carcinosarcoma (UCS).
Methods:
This is a secondary analysis of multicenter retrospective study examining women with stages I-IV UCS who underwent primary surgery. SD was defined as >50% of sarcoma component in uterine tumor. SD patterns were grouped as homologous sarcoma without SD (homo/non-dominance, n = 351), heterologous sarcoma without SD (hetero/non-dominance, n = 174), homologous sarcoma with SD (homo/dominance, n = 175), and heterologous sarcoma with SD (hetero/dominance, n = 189), and correlated to tumor characteristics and survival.
Results:
SD patterns were significantly associated with age, body habitus, carcinoma type, tumor size, depth of myometrial invasion, and nodal metastasis (all, P < 0.05). On univariate analysis, SD was associated with decreased progression-free survival (PFS) and cause-specific survival (CSS) in homologous cases (both, P < 0.05) but not in heterologous cases. On multivariate models, both homologous and heterologous SD patterns remained independent prognostic factors for decreased PFS (adjusted-hazard ratio [HR] ranges: homo/dominance 1.35–1.69, and hetero/dominance 1.47–1.64) and CSS (adjusted-HR ranges: 1.52–1.84 and 1.66–1.81, respectively) compared to homo/non-dominance (all, P < 0.05). Among stage I-III disease, when tumors had SD, adding radiotherapy to chemotherapy was significantly associated with improved PFS (adjusted-HR: homo/dominance 0.49, and hetero/dominance 0.45) and CSS (0.36 and 0.31, respectively) compared to chemotherapy alone (all, P < 0.05); contrary, this association was not observed with absence of SD (all, P > 0.05).
Conclusion:
In UCS, SD impacts survival in homologous but not in heterologous type. Regardless of sarcoma types, SD was associated with decreased survival in UCS; adding radiotherapy to chemotherapy may be an effective postoperative strategy.
Keywords: Uterine carcinosarcoma, Sarcoma dominance, Homologous, Heterologous, Survival
1. Introduction
Uterine carcinosarcoma (UCS) is a rare high-grade endometrial cancer, representing approximately 5% of all endometrial cancers with a gradual increase in its proportion among endometrial cancer over the past few decades [1]. UCS is histologically defined as containing both carcinoma and sarcoma cells in the uterine tumor site [2]. UCS is a biphasic tumor that originally arises in the epithelial carcinoma component, with the subsequent development of a dedifferentiated sarcoma component [2]. This sarcomatous differentiation is best described by the UCS’s unique tumor biology, namely epithelial-mesenchymal transition (EMT) [3,4].
In UCS, the proportion of the dedifferentiated sarcoma component within the primary tumor can exceed the proportion of the primary carcinoma component, a phenomenon called sarcoma dominance (SD). A recent analysis has shown that SD is quite prevalent; it is seen in nearly 40% of UCS and is associated with the heterologous type of sarcoma [5]. Moreover, SD is a prognostic factor for decreased survival in UCS [5]. Collectively, these findings point towards a pivotal role of SD in the tumor biology of UCS.
Regarding a treatment implication of SD in UCS, certain postoperative chemotherapeutic agents are suggested to target the sarcoma component especially in heterologous types, and the use of postoperative radiotherapy may be beneficial in stage I UCS with tumors exhibiting certain factors including SD [5,6]. Despite these suggestive findings regarding SD in UCS, solid evidence remains lacking to outline the impact of SD types in UCS (homologous versus heterologous).
Given the distinctive difference in tumor biology and prognosis between homologous and heterologous uterine sarcomas [7–11], we hypothesize that tumor characteristics and prognoses are different based upon SD patterns in UCS for homologous and heterologous types. The objective of the study is to examine associations of SD pattern and tumor characteristics/survival outcome in women with UCS.
2. Materials and methods
2.1. Database and eligibility
This study was a secondary analysis of a previously organized large-scale multi-center retrospective study from 26 institutions in Japan and the United States. Institutional Review Board approval was obtained at each participating site. This surgical database consisted of consecutive cases of women with stage I-IV UCS who underwent primary hysterectomy-based surgical treatment between 1993 and 2013 with available archived histopathology slides for review [5,6,12–15].
2.2. Clinical information
Variables in this database included patient demographics at diagnosis, tumor characteristics from the surgical specimen, treatment types, and survival outcome. Patient demographics included age, race, country, body mass index (BMI, kg/m2), pregnancy history, history of tamoxifen use, history of pelvic irradiation, and cancer antigen 125 (CA-125, IU/L) levels. Tumor characteristics included carcinoma type, sarcoma type, cancer stage, tumor size, depth of myometrial tumor invasion, presence of SD, lympho-vascular space invasion (LVSI), and lymph node status (pelvic and para-aortic). Treatment characteristics included residual disease status at surgery, and use of postoperative chemotherapy and/or radiotherapy. Survival outcomes included progression-free survival (PFS) and cause-specific survival (CSS).
2.3. Histopathology evaluation
All the specimens were reviewed at each participating institution as described previously [5]. Pathologists who were blinded to clinical information reviewed archived hematoxylin-eosin stained slides and immunohistochemistry results, when available. At the primary tumor site in the hysterectomy specimen, the proportion of sarcoma and carcinoma was scored in a semi-quantified fashion: carcinoma component >50%, sarcoma component >50%, or both components were equal. In addition, carcinoma and sarcoma components as well as histology types at the metastatic sites were also reviewed.
2.4. Study definition
SD was defined as the proportion of the sarcoma component being >50% in the primary tumor within all examined hysterectomy specimens. Based on the combination patterns of sarcoma type (homologous versus heterologous) and presence of SD (yes versus no), the study cohort was grouped into the following four categories: homologous sarcoma without SD (homo/non-dominance), heterologous sarcoma without SD (hetero/non-dominance), homologous sarcoma with SD (homo/dominance), and heterologous sarcoma with SD (hetero/dominance).
The carcinoma component was grouped as low-grade (grade 1–2 endometrioid) or high-grade (grade 3 endometrioid, serous, clear cell, undifferentiated, and mixed), and the sarcoma component was grouped as homologous (endometrial stromal sarcoma, leiomyosarcoma, fibrosarcoma, and undifferentiated sarcoma) or heterologous (rhabdomyosarcoma, osteosarcoma, chondrosarcoma, and liposarcoma) types as previously defined [5].
Cutoff values of patient demographics and tumor characteristics were based on our prior study definition [5]. The 2009 International Federation of Gynecology and Obstetrics (FIGO) system was used to re-classify the cancer stage [16]. Progression-free survival (PFS) was defined as the time interval between the hysterectomy and the first recurrence or progression of disease or death from UCS. Cause-specific survival (CSS) was defined as the time interval between the hysterectomy and the death due to UCS. Cases without these survival events at the last follow-up were censored.
2.5. Statistical considerations
The primary objective of analysis was to examine the association of SD pattern and tumor characteristics. The secondary objective of analysis was to examine the association of SD pattern to survival outcomes (PFS and CSS).
Continuous variables were assessed for normality by means of the Kolmogorov–Smirnov test described as mean (±standard deviation) or median (interquartile range) as appropriate. Statistical differences in continuous variables between groups were assessed by means of one-way ANOVA test or Kruskal-Wallis H test as appropriate. Statistical differences in categorical and ordinal variables between groups were assessed by means of chi-square test as appropriate.
The Kaplan-Meier method was utilized to construct survival curves [17], and the statistical differences between the curves were assessed by means of log-rank test. Cox proportional hazard regression models were used to assess the independent association of SD patterns and survival outcomes (PFS and CSS) on multivariate analysis [18]. In this study, we examined four different adjustment models to examine the association. The purpose of these stepwise models was to assess the independent association in each layer of adjustment: Model 1 adjusted for patient factors alone, Model 2 adjusted for patient factors and surgical factors, Model 3 further adjusted for detailed tumor factors to Model 2, and Model 4 further adjusted for postoperative treatment types to Model 3. Covariates and its cutoff in these models were based on a priori survival factors. Magnitude of the statistical significance was expressed with hazard ratio (HR) and 95% confidence interval (CI).
A sensitivity analysis was performed to examine the association of postoperative therapy and survival based on SD patterns in stage I-III disease. This subgroup was chosen because both chemotherapy and radiotherapy are considered treatment choices after surgery [19].
The variance inflation factor was determined among covariates in multivariate analysis, and a value of ≥2 was defined as multi-collinearity [20]. Over-adjustment was assessed with the ratio of events-of-interest per the entered covariates, and a cutoff level of <10 was interpreted as over-adjustment [21]. A P < 0.05 was considered statistically significant based on two-sided hypothesis tests. Statistical Package for Social Science software (IBM SPSS, version 24.0, Armonk, NY, USA) was used for all the analyses. The STROBE guidelines were followed to outline the results of retrospective observational cohort studies [22].
3. Results
Among 1192 cases of UCS identified, 906 cases were available for histopathology slide review. Of those, 889 cases had evaluation for SD. The most common group was homo/non-dominance (n = 351, 39.5%) followed by hetero/dominance (n = 189, 21.3%), homo/dominance (n = 175, 19.7%) and hetero/non-dominance (n = 174, 19.6%).
Patient demographics are shown in Table 1. On univariate analysis, SD patterns were significantly associated with patient age and body habitus (both, P < 0.05). Specifically, women in the hetero/dominance group were more likely to be older (proportion of ≥60, 76.7%); whereas women in the homo/non-dominance group were younger (59.3%, P < 0.001). Women in the hetero/dominance group had the lowest proportion of obesity (16.1%); whereas women in the homo/dominance group had the highest proportion (31.7%, P = 0.008).
Table 1.
Patient demographics based on sarcoma dominance pattern (N = 889).
| Characteristic |
Homologous/dominance (−) |
Heterologous/dominance (−) |
Homologous/dominance (+) |
Heterologous/dominance (+) |
P-value |
|---|---|---|---|---|---|
| Number | n = 351 | n = 174 | n = 175 | n = 189 | |
| Age (years) | 62 (IQR 14) | 65 (IQR 16) | 64 (IQR 13) | 66 (IQR 14) | <0.001 |
| <60 | 143 (40.7%) | 53 (30.5%) | 52 (29.7%) | 44 (23.3%) | |
| ≥60 | 208 (59.3%) | 121 (69.5%) | 123 (70.3%) | 145 (76.7%) | |
| Race | 0.15 | ||||
| Caucasian | 110 (32.1%) | 60 (34.9%) | 62 (35.8%) | 43 (23.1%) | |
| African | 26 (7.6%) | 21 (12.2%) | 12 (6.9%) | 20 (10.8%) | |
| Hispanic | 9 (2.6%) | 4 (2.3%) | 4 (2.3%) | 5 (2.7%) | |
| Asian | 188 (54.8%) | 82 (47.7%) | 94 (54.3%) | 115 (61.8%) | |
| Others | 10 (2.9%) | 5 (2.9%) | 1 (0.6%) | 3 (1.6%) | |
| Country | 0.09 | ||||
| USA | 168 (47.9%) | 92 (52.9%) | 83 (47.4%) | 75 (39.7%) | |
| Japan | 183 (52.1%) | 82 (47.1%) | 92 (52.6%) | 114 (60.3%) | |
| BMI (kg/m2) | 24.8 (IQR 8.5) | 24.8 (IQR 8.1) | 25.0 (IQR 10.1) | 24.6 (IQR 6.3) | 0.008† |
| <30 | 256 (75.1%) | 123 (76.4%) | 114 (68.3%) | 151 (83.9%) | |
| ≥30 | 85 (24.9%) | 38 (23.6%) | 53 (31.7%) | 29 (16.1%) | |
| Gravida | 0.31 | ||||
| Nulli | 64 (18.7%) | 27 (16.1%) | 23 (13.7%) | 24 (13.1%) | |
| Multi | 279 (81.3%) | 141 (83.9%) | 145 (86.3%) | 159 (86.9%) | |
| History of tamoxifen use | 0.30 | ||||
| No | 333 (96.2%) | 165 (94.8%) | 163 (93.7%) | 175 (92.6%) | |
| Yes | 13 (3.8%) | 9 (5.2%) | 11 (6.3%) | 14 (7.4%) | |
| Prior pelvic irradiation | 0.98 | ||||
| No | 346 (98.6%) | 172 (98.9%) | 173 (98.9%) | 186 (98.4%) | |
| Yes | 5 (1.4%) | 2 (1.1%) | 2 (1.1%) | 3 (1.6%) | |
| Preop CA-125 (IU/L) | 21 (IQR 46) | 31 (IQR 61) | 22 (IQR 33) | 23 (IQR 52) | 0.51 |
| <30 | 143 (40.7%) | 53 (30.5%) | 72 (41.1%) | 82 (43.4%) | |
| ≥30 | 95 (27.1%) | 59 (33.9%) | 48 (27.4%) | 54 (28.6%) | |
| Not assessed | 113 (32.2%) | 62 (35.6%) | 55 (31.4%) | 53 (28.0%) | |
| Residual disease | 0.29 | ||||
| No | 313 (90.2%) | 140 (85.9%) | 155 (90.6%) | 151 (86.3%) | |
| Yes | 34 (9.8%) | 23 (14.1%) | 16 (9.4%) | 24 (13.7%) | |
| Postop radiotherapy | 0.67 | ||||
| None | 253 (72.7%) | 126 (74.1%) | 130 (74.7%) | 148 (79.1%) | |
| VBT alone | 12 (3.4%) | 4 (2.4%) | 3 (1.7%) | 5 (2.7%) | |
| WPRT ± VBT | 83 (23.9%) | 40 (23.5%) | 41 (23.6%) | 34 (18.2%) | |
| Postop chemotherapy | 0.73 | ||||
| No | 108 (31.0%) | 61 (35.7%) | 55 (31.6%) | 63 (33.7%) | |
| Yes | 240 (69.0%) | 110 (64.0%) | 119 (68.4%) | 124 (66.3%) | |
Median (IQR) or number (percent per column) is shown. Significant P-values are emboldened.
among available cases.
comparison of BMI ≥30.
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; VBT, vaginal brachytherapy; and WPRT, whole pelvic radiotherapy.
Tumor characteristics are shown in Table 2. On univariate analysis, carcinoma type, tumor size, depth of myometrial tumor invasion, and nodal metastasis patterns were significantly associated with SD patterns (all, P < 0.05). First, the sarcoma dominant groups had a higher proportion of serous histology (22.3–28.0% versus 14.4–15.7%) and a lower proportion of grade 3 endometrioid histology (20.1–23.4% versus 25.9–30.2%) compared to the non-dominant groups (P < 0.001). The sarcoma dominant groups had a disproportionally higher incidence of large tumor size (≥10cm) compared to the non-dominant groups (19.4–24.7% versus 6.1–11.2%, P < 0.001).
Table 2.
Tumor characteristics based on sarcoma dominance pattern (N = 889).
| Characteristic |
Homologous/dominance (−) |
Heterologous/dominance (−) |
Homologous/dominance (+) |
Heterologous/dominance (+) |
P-value |
|---|---|---|---|---|---|
| Number | n = 351 | n = 174 | n = 175 | n = 189 | |
| Carcinoma type | 0.025 | ||||
| Low-grade | 96 (27.4%) | 40 (23.0%) | 65 (37.1%) | 52 (27.5%) | |
| High-grade | 255 (72.6%) | 134 (77.0%) | 110 (62.9%) | 137 (72.5%) | |
| Carcinoma component | <0.001 | ||||
| Grade 1 endometrioid | 37 (10.5%) | 22 (12.6%) | 30 (17.1%) | 26 (13.8%) | |
| Grade 2 endometrioid | 59 (16.8%) | 18 (10.3%) | 35 (20.0%) | 26 (13.8%) | |
| Grade 3 endometrioid | 106 (30.2%) | 45 (25.9%) | 41 (23.4%) | 38 (20.1%) | |
| Serous | 55 (15.7%) | 25 (14.4%) | 39 (22.3%) | 53 (28.0%) | |
| Clear cell | 7 (2.0%) | 3 (1.7%) | 3 (1.7%) | 3 (1.6%) | |
| Undifferentiated | 9 (2.6%) | 5 (2.9%) | 6 (3.4%) | 11 (5.8%) | |
| Mixed | 77 (21.9%) | 55 (31.6%) | 18 (10.3%) | 30 (15.9%) | |
| Others | 1 (0.3%) | 1 (0.6%) | 3 (1.7%) | 2 (1.1%) | |
| Stage | 0.39 | ||||
| I | 183 (52.1%) | 76 (43.7%) | 84 (48.0%) | 94 (49.7%) | |
| II | 31 (8.8%) | 10 (5.7%) | 9 (5.1%) | 14 (7.4%) | |
| III | 95 (27.1%) | 64 (36.8%) | 58 (33.1%) | 54 (28.6%) | |
| IV | 42 (12.0%) | 24 (13.8%) | 24 (13.7%) | 27 (14.3%) | |
| Tumor size (cm) | <0.001 | ||||
| <5 | 159 (46.4%) | 59 (34.9%) | 47 (27.6%) | 48 (26.4%) | |
| 5–9.9 | 163 (47.5%) | 91 (53.8%) | 90 (52.9%) | 89 (48.9%) | |
| ≥10 | 21 (6.1%) | 19(11.2%) | 33 (19.4%) | 45 (24.7%) | |
| Myometrial invasion | 0.035 | ||||
| Inner-half | 185 (53.0%) | 78 (45.1%) | 84 (48.6%) | 112 (59.6%) | |
| Outer-half | 164 (47.0%) | 95 (54.9%) | 89 (51.4%) | 76 (40.4%) | |
| LVSI | 0.45 | ||||
| No | 133 (38.0%) | 63 (36.2%) | 74 (42.5%) | 81 (42.9%) | |
| Yes | 217 (62.0%) | 111 (63.8%) | 100 (57.5%) | 108 (57.1%) | |
| PLN metastasis | 0.001 | ||||
| No | 210 (59.8%) | 79 (45.4%) | 98 (56.0%) | 94 (49.7%) | |
| Yes | 66 (18.8%) | 48 (27.6%) | 26 (14.9%) | 30 (15.9%) | |
| Not assessed | 75 (21.4%) | 47 (27.0%) | 51 (29.1%) | 65 (34.4%) | |
| PAN metastasis | 0.039 | ||||
| No | 142 (40.5%) | 47 (27.0%) | 64 (36.6%) | 60 (31.7%) | |
| Yes | 31 (8.8%) | 21 (12.1%) | 14 (8.0%) | 13 (6.9%) | |
| Not assessed | 178 (50.7%) | 106 (60.9%) | 97 (55.4%) | 116 (61.4%) | |
| Lymph node ratio (%)† | |||||
| PLN | 11.1 (IQR 27.6) | 23.9 (IQR 43.7) | 20.2 (IQR 47.6) | 13.2 (IQR 23.6) | 0.18 |
| PAN | 45.0 (IQR 83.5) | 64.3 (IQR 69.6) | 33.3 (IQR 58.7) | 20.0 (IQR 61.7) | 0.09 |
| Cervical stroma pattern* | <0.001 | ||||
| Carcinoma only | 73 (92.4%) | 33 (86.8%) | 12 (31.6%) | 16 (40%) | |
| Sarcoma only | 2 (2.5%) | 0 | 22 (57.9%) | 20 (50%) | |
| Carcinoma/sarcoma | 4 (5.1%) | 5 (13.2%) | 4 (10.5%) | 4 (10%) | |
| Adnexal pattern* | <0.001 | ||||
| Carcinoma only | 38 (80.9%) | 22 (73.3%) | 10 (37.0%) | 19 (67.9%) | |
| Sarcoma only | 1 (2.1%) | 1 (2.1%) | 11 (40.7%) | 7 (25.0%) | |
| Carcinoma/sarcoma | 8 (17.0%) | 7 (23.3%) | 6 (22.2%) | 2 (7.1%) | |
| Lymph node pattern** | <0.001 | ||||
| Carcinoma only | 56 (91.8%) | 36 (85.7%) | 16 (64%) | 15 (57.7%) | |
| Sarcoma only | 1 (1.6%) | 2 (4.8%) | 8 (32%) | 9 (34.6%) | |
| Carcinoma/sarcoma | 4 (6.6%) | 4 (9.5%) | 1 (4%) | 2 (7.7%) | |
| Omentum pattern* | 0.009 | ||||
| Carcinoma only | 13 (76.5%) | 9 (89.2%) | 3 (25%) | 10 (90.9%) | |
| Sarcoma only | 1 (5.9%) | 0 | 4 (33.3%) | 0 | |
| Carcinoma/sarcoma | 3 (17.6%) | 4 (30.8%) | 5 (41.7%) | 1 (9.1%) | |
Median (IQR) or number (percent per column) is shown. Significant P-values are emboldened.
among node-involved cases,
among available results for histology types,
PLN and/or PAN cases with available results for histology types.
Abbreviations: LVSI, lympho-vascular space invasion; PLN, pelvic lymph node; and PAN, para-aortic lymph node.
Among the groups, tumors with heterologous SD were least likely to have deep myometrial invasion (40.4% versus 47.0–54.9%, P = 0.035). The sarcoma dominant groups had a lower proportion of nodal metastasis compared to the non-dominant groups: pelvic (14.9–15.9% versus 18.8–27.6%) and para-aortic (6.9–8.0% versus 8.8–12.1%) (both, P < 0.05). Among cases with known histology types in the extra-uterine sites, metastatic tumors were more likely to have sarcoma cells when uterine tumors had SD: cervical stroma (60–68.4% versus 7.6–13.2%), adnexa (32.1–62.9% versus 19.1–25.4%), lymph nodes (36.0–41.3% versus 8.2–14.3%), and omentum (9.1–75% versus 23.5–30.8%) (all, P < 0.05).
The median follow-up time of censored cases was 38.6 (interquartile rage 12.8) months. There were 419 survival events for recurrence/progression of disease or death due to UCS. On univariate analysis, SD patterns were significantly associated with PFS (Fig. 1A, P = 0.001) and CSS (Fig. 1B, P = 0.001). The 5-year PFS rates were 53.8% for homo/non-dominance, 37.4% for hetero/non-dominance, 43.6% for homo/dominance, and 39.6% for hetero/dominance, respectively; and the 5-year CSS rates were 68.2%, 56.1%, 51.7%, and 48.6%, respectively.
Fig. 1.
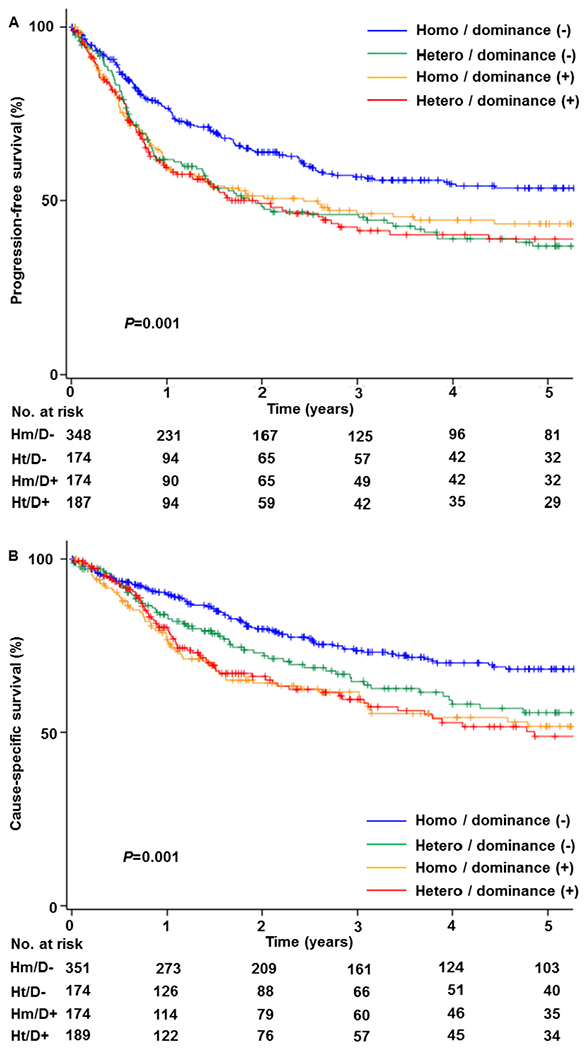
Survival outcome (N = 889). Log-rank test for P-values. A) Progression-free survival and B) cause-specific survival. Abbreviations: dominance, sarcoma dominance; homo, homologous sarcoma; and hetero, heterologous sarcoma.
In a pairwise comparison, survival outcome was compared between SD and non-dominance stratified by the sarcoma type. Among 526 homologous sarcoma cases, presence of SD was significantly associated with decreased PFS (unadjusted-HR 1.48, 95%CI 1.13–1.93, P = 0.004) and CSS (unadjusted-HR 1.67, 95%CI 1.22–2.28, P = 0.001). However, among 363 heterologous sarcoma cases, presence of SD was not associated with PFS and CSS (both, P > 0.05).
When the association of SD patterns and survival was adjusted on various multivariate models (Table 3), both homologous and heterologous SD patterns remained independent prognostic factors for decreased PFS (adjusted-HR ranges: homo/dominance 1.35–1.69, and hetero/dominance 1.47–1.64) and CSS (adjusted-HR ranges: 1.52–1.84 and 1.66–1.81, respectively) compared to homo/non-dominance (all, P < 0.05). The hetero/non-dominance group was also associated with decreased PFS compared to the homo/non-dominance group (adjusted-HR ranges 1.37–1.49, P < 0.05).
Table 3.
Multivariate models for survival outcome (N = 889).
| Adjustment model | Progression-free survival |
Cause-specific survival |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Unadjusted | ||||
| Homologous/dominance (−) | 1 | 1 | ||
| Heterologous/dominance (−) | 1.55 (1.19–2.01) | 0.001 | 1.38 (1.00–1.91) | 0.05 |
| Homologous/dominance (+) | 1.47 (1.13–1.93) | 0.005 | 1.67 (1.22–2.28) | 0.001 |
| Heterologous/dominance (+) | 1.53 (1.18–1.99) | 0.001 | 1.70 (1.25–2.31) | 0.001 |
| Patient demographics | ||||
| Homologous/dominance (−) | 1 | 1 | ||
| Heterologous/dominance (−) | 1.49 (1.15–1.93) | 0.003 | 1.32 (0.96–1.82) | 0.092 |
| Homologous/dominance (+) | 1.35 (1.04–1.77) | 0.027 | 1.52 (1.11–2.09) | 0.009 |
| Heterologous/dominance (+) | 1.47 (1.13–1.91) | 0.005 | 1.66 (1.22–2.26) | 0.001 |
| Patient demographics, surgical factors | ||||
| Homologous/dominance (−) | 1 | 1 | ||
| Heterologous/dominance (−) | 1.42 (1.09–1.86) | 0.01 | 1.34 (0.96–1.86) | 0.08 |
| Homologous/dominance (+) | 1.49 (1.13–1.97) | 0.004 | 1.75 (1.27–2.42) | 0.001 |
| Heterologous/dominance (+) | 1.57 (1.20–2.07) | 0.001 | 1.73 (1.26–2.38) | 0.001 |
| Patient demographics, surgical factors, detailed tumor characteristics | ||||
| Homologous/dominance (−) | 1 | 1 | ||
| Heterologous/dominance (−) | 1.37 (1.04–1.80) | 0.025 | 1.25 (0.89–1.74) | 0.19 |
| Homologous/dominance (+) | 1.57 (1.18–2.08) | 0.002 | 1.73 (1.24–2.40) | 0.001 |
| Heterologous/dominance (+) | 1.64 (1.23–2.19) | 0.001 | 1.81 (1.30–2.51) | <0.001 |
| Patient demographics, surgical factors, detailed tumor characteristics, postop treatment types | ||||
| Homologous/dominance (−) | 1 | 1 | ||
| Heterologous/dominance (−) | 1.16 (0.88–1.54) | 0.30 | 1.02 (0.72–1.44) | 0.91 |
| Homologous/dominance (+) | 1.69 (1.27–2.24) | <0.001 | 1.84 (1.32–2.57) | <0.001 |
| Heterologous/dominance (+) | 1.64 (1.23–2.20) | 0.001 | 1.66 (1.18–2.32) | 0.004 |
Cox proportional hazard regression models for P-values. Significant P-values were emboldened. Patient demographics included age (<60 versus ≥60) and country (USA versus Japan). Surgical factors included residual disease at surgery (yes versus no) and caner stage (I versus II-IV). Detailed tumor factors included carcinoma component (low-grade versus high-grade), tumor size (<5 versus ≥5 cm), lympho-vascular space invasion (presence versus absence), and depth of myometrial invasion (inner-half versus outer-half). Lymph node status was not chosen as multicollinearity to stage. Postoperative treatment types included radiotherapy (yes versus no) and chemotherapy (yes versus no). Abbreviations: HR, hazard ratio; CI, confidence interval; and dominance, sarcoma dominance.
There were 772 cases of stage I-III disease examined for post-operative therapy based on SD patterns. When tumors had SD, postoperative radiotherapy was significantly associated with improved PFS (adjusted-HR: 0.49 for homo/dominance, and 0.36 for hetero/dominance) and CSS (adjusted-HR: 0.37 for homo/dominance, and 0.35 for hetero/dominance) regardless of sarcoma types (all, P < 0.05; Table 4). When tumors did not have SD, postoperative radiotherapy was not associated with PFS and CSS (all, P > 0.05).
Table 4.
Effects of postoperative radiotherapy on survival based on sarcoma dominance patterns for stage I-III disease (n = 772).
| Characteristic | Progression-free survival |
Cause-specific survival |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Homologous/dominance (−) | ||||
| Radiotherapy (−) | 1 | 1 | ||
| Radiotherapy (+) | 0.90 (0.60–1.36) | 0.62 | 1.02 (0.63–1.68) | 0.92 |
| Heterologous/dominance (−) | ||||
| Radiotherapy (−) | 1 | 1 | ||
| Radiotherapy (+) | 0.74 (0.44–1.24) | 0.25 | (0.29–1.26) | 0.17 |
| Homologous/dominance (+) | ||||
| Radiotherapy (−) | 1 | 1 | ||
| Radiotherapy (+) | 0.49 (0.26–0.92) | 0.026 | 0.37 (0.16–0.86) | 0.021 |
| Heterologous/dominance (+) | ||||
| Radiotherapy (−) | 1 | 1 | ||
| Radiotherapy (+) | 0.36 (0.18–0.70) | 0.003 | 0.35 (0.16–0.77) | 0.009 |
Cox proportional hazard regression test for P-values (adjusted for age, stage, and chemotherapy use). Significant P-values are emboldened. Abbreviations: HR, hazard ratio; and CI, confidence interval.
Similarly, when tumors had SD, adding radiotherapy to chemotherapy was significantly associated with improved PFS (adjusted-HR: homo/dominance 0.49, and hetero/dominance 0.45) and CSS (adjusted-HR: 0.36 for homo/dominance, and 0.31 for hetero/dominance) compared to chemotherapy alone (all, P < 0.05; Table 5); contrary, this association was not observed when tumors had no SD (all, P > 0.05).
Table 5.
Effects of postoperative treatment type on survival based on sarcoma dominance patterns for stage I-III disease (n = 772).
| Characteristic | Progression-free survival |
Cause-specific survival |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Homologous/dominance (−) | ||||
| Chemotherapy alone | 1 | 1 | ||
| Radiotherapy alone | 2.23 (1.19–4.19) | 0.013 | 2.74 (1.25–6.01) | 0.012 |
| Chemotherapy/radiotherapy | 0.92 (0.54–1.57) | 0.76 | 1.38 (0.72–2.66) | 0.33 |
| None | 2.64 (1.61–4.33) | <0.001 | 4.46 (2.40–8.30) | <0.001 |
| Heterologous/dominance (−) | ||||
| Chemotherapy alone | 1 | 1 | ||
| Radiotherapy alone | 1.15 (0.53–2.52) | 0.72 | 0.76 (0.22–2.63) | 0.66 |
| Chemotherapy/radiotherapy | 0.73 (0.37–1.44) | 0.37 | 0.75 (0.30–1.86) | 0.54 |
| None | 1.50 (0.86–2.61) | 0.16 | 1.70 (0.82–3.52) | 0.16 |
| Homologous/dominance (+) | ||||
| Chemotherapy alone | 1 | 1 | ||
| Radiotherapy alone | 1.44 (0.44–4.74) | 0.55 | 0.82 (0.11–6.13) | 0.85 |
| Chemotherapy/radiotherapy | 0.44 (0.21–0.90) | 0.025 | 0.36 (0.14–0.94) | 0.037 |
| None | 1.40 (0.76–2.60) | 0.28 | 1.43 (0.73–2.81) | 0.30 |
| Heterologous/dominance (+) | ||||
| Chemotherapy alone | 1 | 1 | ||
| Radiotherapy alone | 1.68 (0.40–7.06) | 0.48 | 2.80 (0.82–9.50) | 0.10 |
| Chemotherapy/radiotherapy | 0.45 (0.21–0.98) | 0.045 | 0.31 (0.11–0.89) | 0.029 |
| None | 3.64 (1.98–6.69) | <0.001 | 3.19 (1.60–6.34) | 0.001 |
Cox proportional hazard regression models for P-values (adjusted for age and stage). Significant P-values are emboldened. Abbreviations: HR, hazard ratio; and CI, confidence interval.
4. Discussion
SD is an important pathological factor that impacts both treatment and outcome of UCS. Salient findings from this study are that tumor characteristics for SD in UCS include: serous histology, large tumor size, and less lymph node invasion. The proportion of sarcoma component is also a prognostic factor in UCS, particularly in the homologous type. Lastly, when UCS tumors exhibit a large fraction of sarcoma, radiotherapy seems to enhance postoperative treatment.
The exact mechanism by which the sarcoma component becomes a dominant element in the uterine tumor site remains unknown. This phenomenon may be related to EMT. That is, the tumor volume of the sarcoma component reflects the extent and severity of EMT occurring in the tumor, and the UCS tumors with SD may reflect accelerated EMT with enhanced sarcomatous dedifferentiation from the primary carcinoma components.
Recent high-throughput molecular analyses have shown that UCS tumors with heterologous dedifferentiation have higher EMT activity compared to their homologous counterparts [3]. These findings partly support our prior observations that UCS with a heterologous sarcoma component has a higher incidence of SD compared to UCS with a homologous sarcoma component (50.6–56.5% versus 30.1–40.4%) [5]. Therefore, these results imply that UCS with SD may possess accelerated EMT activity resulting in different tumor characteristics and prognosis.
If the accelerated EMT phenomenon is in fact the mechanism for SD in UCS, targeting EMT signaling may be an attractive treatment approach because prognosis of women with UCS remains poor with current available treatment strategies. Various target markers have been identified in EMT signaling in endometrial cancers including UCS with possible future implications for cancer treatment [3,23–27]. Moreover, we found that older age and large body habitus are suggestive for SD in our study. Thus, it may be of interesting how aging and obesity impact EMT development.
This study found that the prognosis of women in the heterologous group was worse than those in the homologous group for tumors without SD. For sarcoma dominant cases, survival was similar between the homologous and heterologous groups. This clearly indicates that presence of SD impacts survival more in homologous type than heterologous type. A possible explanation of this observation is the degree of EMT activity, generally reflecting aggressive tumor biology [3,28], is generally high in heterologous type UCS even when the sarcomatous component is small [3]. Thus, attention might be warranted in homologous type UCS when evaluating SD given that survival is distinctive based on the proportion of the sarcomatous component.
It may be useful to consider SD when planning treatment for UCS because SD corresponds with prognostic factors such as loco-regional tumor metastasis with sarcoma, response to antisarcoma agents, and recurrence with sarcoma [5,6]. This study suggests that regardless of sarcoma type, UCS with SD is more sensitive to radiotherapy compared to UCS without SD. This observation seems consistent with a recent meta-analysis reporting the effectiveness of postoperative radiotherapy for uterine sarcoma [29], and supports the concept that sarcoma dominant UCS tumors clinically behave more like sarcoma than carcinoma.
A strength of this investigation is that it is the first in-depth study of the impact of SD in UCS. The sample size is also one of the largest reported in the literature. A comprehensive histopathology slide review enhances the quality of the study. In general, UCS is rare tumor and is routinely excluded from clinical trials. Thus, there has been relatively little data from a large group such as this study to help guide therapy, thus highlighting the value of our results.
There are several study limitations. First, as a retrospective study, it is vulnerable to unanticipated confounding factors in the analysis. For example, we are not able to retrieve information regarding the choice for postoperative therapy. In addition, the majority of the study population is Asian, and thus, generalizability to other populations remains unknown. Lastly, central pathology review was not performed to confirm the SD; therefore, interobserver agreement and reproducibility among the pathologists remain undetermined. Unlike uterine adenosarcoma where sarcomatous overgrowth (>25%) is well defined [30], the cutoff of >50% for the sarcoma component is arbitrarily defined in our study. It remains unknown if different cutoff for the proportion of the sarcoma component will produce similar results, particularly in the heterologous type. Last, while EMT is suggestive to link SD in UCS, there is no actual translational research in this study.
Clinical utilities of the study argue for the routine description of the proportion of carcinoma and sarcoma components in the synoptic report in UCS. Based on our findings, we respectfully suggest that the addition of radiotherapy to systemic chemotherapy may be an effective postoperative strategy to reduce the risk of disease recurrence and mortality in stage I-III UCS with SD as it is the factor for local expansion rather than distant metastasis. Further study with a prospective design is necessary to confirm this finding.
Acknowledgments
Funding support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Disclosure statement
There is no conflict of interest in all the authors for this study.
References
- [1].Matsuo K, Ross MS, Machida H, Blake EA, Roman LD, Trends of uterine carcinosarcoma in the United States, J. Gynecol. Oncol 29 (2018), e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cantrell LA, Blank SV, Duska LR, Uterine carcinosarcoma: a review of the literature, Gynecol. Oncol 137 (2015) 581–588. [DOI] [PubMed] [Google Scholar]
- [3].Cherniack AD, Shen H, Walter V, Stewart C, Murray BA, Bowlby R, Hu X, Ling S, Soslow RA, Broaddus RR, Zuna RE, Robertson G, Laird PW, Kucherlapati R, Mills GB, Weinstein JN, Zhang J, Akbani R, Levine DA, Integrated molecular characterization ofuterine carcinosarcoma, Canc. Cell 31 (2017) 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhao S, Bellone S, Lopez S, Thakral D, Schwab C, English DP, Black J, Cocco E, Choi J, Zammataro L, Predolini F, Bonazzoli E, Bi M, Buza N, Hui P, Wong S, Abu-Khalaf M, Ravaggi A, Bignotti E, Bandiera E, Romani C, Todeschini P, Tassi R, Zanotti L, Odicino F, Pecorelli S, Donzelli C, Ardighieri L, Facchetti F, Falchetti M, Silasi DA, Ratner E, Azodi M, Schwartz PE, Mane S, Angioli R, Terranova C, Quick CM, Edraki B, Bilguvar K, Lee M, Choi M, Stiegler AL, Boggon TJ, Schlessinger J, Lifton RP, Santin AD, Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition, Proc. Natl. Acad. Sci. U. S. A 113 (2016) 12238–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Matsuo K, Takazawa Y, Ross MS, Elishaev E, Podzielinski I, Yunokawa M, Sheridan TB, Bush SH, Klobocista MM, Blake EA, Takano T, Matsuzaki S, Baba T, Satoh S, Shida M, Nishikawa T, Ikeda Y, Adachi S, Yokoyama T, Takekuma M, Fujiwara K, Hazama Y, Kadogami D, Moffitt MN, Takeuchi S, Nishimura M, Iwasaki K, Ushioda N, Johnson MS, Yoshida M, Hakam A, Li SW, Richmond AM, Machida H, Mhawech-Fauceglia P, Ueda Y, Yoshino K, Yamaguchi K, Oishi T, Kajiwara H, Hasegawa K, Yasuda M, Kawana K, Suda K, Miyake TM, Moriya T, Yuba Y, Morgan T, Fukagawa T, Wakatsuki A, Sugiyama T, Pejovic T, Nagano T, Shimoya K, Andoh M, Shiki Y, Enomoto T, Sasaki T, Mikami M, Shimada M, Konishi I, Kimura T, Post MD, Shahzad MM, Im DD, Yoshida H, Omatsu K, Ueland FR, Kelley JL, Karabakhtsian RG, Roman LD, Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma, Ann. Oncol 27 (2016) 1257–1266. [DOI] [PubMed] [Google Scholar]
- [6].Matsuo K, Omatsu K, Ross MS, Johnson MS, Yunokawa M, Klobocista MM, Im DD, Bush SH, Ueda Y, Takano T, Blake EA, Hasegawa K, Baba T, Shida M, Satoh S, Yokoyama T, Machida H, Adachi S, Ikeda Y, Iwasaki K, Miyake TM, Yanai S, Nishimura M, Nagano T, Takekuma M, Takeuchi S, Pejovic T, Shahzad MM, Ueland FR, Kelley JL, Roman LD, Impact of adjuvant therapy on recurrence patterns in stage I uterine carcinosarcoma, Gynecol. Oncol 145 (2017) 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fadare O, Heterologous and rare homologous sarcomas of the uterine corpus: a clinicopathologic review, Adv. Anat. Pathol 18 (2011) 60–74. [DOI] [PubMed] [Google Scholar]
- [8].Pinto A, Kahn RM, Rosenberg AE, Slomovitz B, Quick CM, Whisman MK, Huang M, Uterine rhabdomyosarcoma in adults, Hum. Pathol 74 (2018) 122–128. [DOI] [PubMed] [Google Scholar]
- [9].Su M, Tokairin T, Nishikawa Y, Yoshioka T, Takahashi O, Watanabe H, Doi Y, Omori Y, Sageshima M, Tanaka T, Enomoto K, Primary osteosarcoma of the uterine corpus: case report and review of the literature, Pathol. Int 52 (2002) 158–163. [DOI] [PubMed] [Google Scholar]
- [10].Namizato CS, Muriel-Cueto P, Baez-Perez JM, Bartha JL, Hervias-Vivancos B, Chondrosarcoma of the uterus: case report and literature review, Arch. Gynecol. Obstet 278 (2008) 369–372. [DOI] [PubMed] [Google Scholar]
- [11].Levine PH, Wei XJ, Gagner JP, Flax H, Mittal K, Blank SV, Pleomorphic liposarcoma of the uterus: case report and literature review, Int. J. Gynecol. Pathol 22 (2003) 407–411. [DOI] [PubMed] [Google Scholar]
- [12].Matsuo K, Johnson MS, Im DD, Ross MS, Bush SH, Yunokawa M, Blake EA, Takano T, Klobocista MM, Hasegawa K, Ueda Y, Shida M, Baba T, Satoh S, Yokoyama T, Machida H, Ikeda Y, Adachi S, Miyake TM, Iwasaki K, Yanai S, Takeuchi S, Nishimura M, Nagano T, Takekuma M, Shahzad MMK, Pejovic T, Omatsu K, Kelley JL, Ueland FR, Roman LD, Survival outcome of women with stage IV uterine carcinosarcoma who received neoadjuvant chemotherapy followed by surgery, J. Surg. Oncol 117 (2018) 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Matsuo K, Ross MS, Bush SH, Yunokawa M, Blake EA, Takano T, Ueda Y, Baba T, Satoh S, Shida M, Ikeda Y, Adachi S, Yokoyama T, Takekuma M, Takeuchi S, Nishimura M, Iwasaki K, Yanai S, Klobocista MM, Johnson MS, Machida H, Hasegawa K, Miyake TM, Nagano T, Pejovic T, Shahzad MM, Im DD, Omatsu K, Ueland FR, Kelley JL, Roman LD, Tumor characteristics and survival outcomes of women with tamoxifen-related uterine carcino-sarcoma, Gynecol. Oncol 144 (2017) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matsuo K, Ross MS, Im DD, Klobocista MM, Bush SH, Johnson MS, Takano T, Blake EA, Ikeda Y, Nishimura M, Ueda Y, Shida M, Hasegawa K, Baba T, Adachi S, Yokoyama T, Satoh S, Machida H, Yanai S, Iwasaki K, Miyake TM, Takeuchi S, Takekuma M, Nagano T, Yunokawa M, Pejovic T, Omatsu K, Shahzad MMK, Kelley JL, Ueland FR, Roman LD, Significance of venous thromboembolism in women with uterine carcinosarcoma, Gynecol. Oncol 148 (2018) 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Matsuo K, Ross MS, Yunokawa M, Johnson MS, Machida H, Omatsu K, Klobocista MM, Im DD, Satoh S, Baba T, Ikeda Y, Bush SH, Hasegawa K, Blake EA, Takekuma M, Shida M, Nishimura M, Adachi S, Pejovic T, Takeuchi S, Yokoyama T, Ueda Y, Iwasaki K, Miyake TM, Yanai S, Nagano T, Takano T, Shahzad MMK, Ueland FR, Kelley JL, Roman LD, Salvage chemotherapy with taxane and platinum for women with recurrent uterine carcinosarcoma, Gynecol. Oncol 147 (2017) 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int. J. Gynaecol. Obstet 105 (2009) 103–104. [DOI] [PubMed] [Google Scholar]
- [17].Kaplan EL, Meier P, Nonparametric estimation from incomplete observations, J. Am. Stat. Assoc 53 (1958) 457–481. [Google Scholar]
- [18].Cox DR, Regression models and life-tables, J. R Stat. Soc. Ser. B Stat. Methodol 34 (1972) 187–220. [Google Scholar]
- [19].Uterine Neoplasms, NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines), 2018. http://www.nccn.org/. (Accessed 1 January 2018).
- [20].Mansfield ER, Helms BP, Detecting multicollinearity, Am. Stat 36 (1982) 158–160. [Google Scholar]
- [21].Schisterman EF, Cole SR, Platt RW, Overadjustment bias and unnecessary adjustment in epidemiologic studies, Epidemiology 20 (2009) 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Colas E, Pedrola N, Devis L, Ertekin T, Campoy I, Martinez E, Llaurado M, Rigau M, Olivan M, Garcia M, Cabrera S, Gil-Moreno A, Xercavins J, Castellvi J, Garcia A, Ramon y Cajal S, Moreno-Bueno G, Dolcet X, Alameda F, Palacios J, Prat J, Doll A, Matias-Guiu X, Abal M, Reventos J, The EMT signaling pathways in endometrial carcinoma, Clin. Transl. Oncol 14 (2012) 715–720. [DOI] [PubMed] [Google Scholar]
- [24].Carvalho MJ, Laranjo M, Abrantes AM, Torgal I, Botelho MF, Oliveira CF, Clinical translation for endometrial cancer stem cells hypothesis, Canc. Metastasis Rev 34 (2015) 401–416. [DOI] [PubMed] [Google Scholar]
- [25].Kent CN, Guttilla Reed IK, Regulation of epithelial-mesenchymal transition in endometrial cancer: connecting PI3K, estrogen signaling, and microRNAs, Clin. Transl. Oncol 18 (2016) 1056–1061. [DOI] [PubMed] [Google Scholar]
- [26].Wik E, Raeder MB, Krakstad C, Trovik J, Birkeland E, Hoivik EA, Mjos S, Werner HM, Mannelqvist M, Stefansson IM, Oyan AM, Kalland KH, Akslen LA, Salvesen HB, Lack of estrogen receptor-alpha is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma, Clin. Canc. Res 19 (2013) 1094–1105. [DOI] [PubMed] [Google Scholar]
- [27].Muinelo-Romay L, Colas E, Barbazan J, Alonso-Alconada L, Alonso-Nocelo M, Bouso M, Curiel T, Cueva J, Anido U, Forteza J, Gil-Moreno A, Reventos J, Lopez-Lopez R, Abal M, High-risk endometrial carcinoma profiling identifies TGF-beta 1 as a key factor in the initiation of tumor invasion, Mol. Canc. Therapeut 10 (2011) 1357–1366. [DOI] [PubMed] [Google Scholar]
- [28].Integrated genomic and molecular characterization of cervical cancer, Nature 543 (2017) 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sampath S, Gaffney DK, Role of radiotherapy treatment of uterine sarcoma, Best Pract. Res. Clin. Obstet. Gynaecol 25 (2011) 761–772. [DOI] [PubMed] [Google Scholar]
- [30].Machida H, Nathenson MJ, Takiuchi T, Adams CL, Garcia-Sayre J, Matsuo K, Significance of lymph node metastasis on survival of women with uterine adenosarcoma, Gynecol. Oncol 144 (2017) 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]


