Abstract
Objective
To describe how population-based statistics for rare epithelial ovarian cancers are evolving.
Methods
This is a retrospective observational study examining the Surveillance, Epidemiology, and End Results Program from 1988 to 2016. Overall survival (OS) of clear cell (OCCC), mucinous (MOC), and low-grade serous (LGSOC) ovarian cancers were compared to high-grade serous ovarian cancer (HGSOC) by fitting a propensity score matching.
Results
Among 113,365 ovarian malignancies, 5780 OCCCs (5.1%), 7561 MOCs (6.7%), and 2021 LGSOCs (1.8%) were compared to 38,199 HGSOCs. OCCCs and MOCs were more likely to be diagnosed with stage I disease compared to HGSOC (57.0–59.5% versus 8.6%, P < 0.001). For early-stage disease, OCCC (hazard ratio [HR] 0.91, 95% confidence interval [CI] 0.82–1.01) and MOC (HR 0.94, 95% CI 0.85–1.04) had similar OS to HGSOC whereas LGSOC had superior OS (HR 0.93, 95% CI 0.89–0.97) versus HGSOC. Conversely, for advanced-stage disease, OCCC (HR 1.42, 95% CI 1.32–1.53) and MOC (HR 1.11, 95% CI 1.09–1.13) had poorer OS whereas LGSOC (HR 0.86, 95% CI 0.84–0.89) had superior OS compared to HGSOC. OCCC (HR range, 1.92–2.45) and MOC (HR range, 1.73–2.22) had particularly poorer OS in the first three years following diagnosis compared to HGSOC. Population-level statistics for advanced-stage disease showed that 5-year OS rates have increased in HGSOC (16.9% to 36.8%, P < 0.001) and LGSOC (50.8% to 66.4%, P = 0.010); but remain unchanged for OCCC (21.0% to 28.2%, P = 0.174) and MOC (21.4% to 16.5%, P = 0.102).
Conclusion
OCCC, MOC, and LGSOC comprise 2–7% of ovarian malignancies, have distinct characteristics and survival compared to HGSOC. While these rare tumors have a favorable to comparable prognosis in early-stage disease, disproportionally poor survival in advanced-stage OCCC and MOC highlights the need for further research into novel treatment strategies.
Keywords: Ovarian cancer, Clear cell, Mucinous, Low-grade serous, Rare tumor, Survival
1. Introduction
While the incidence of ovarian cancer has been steadily decreasing over the past few decades, it remains the most deadly gynecologic malignancy in the United States [1,2]. In 2019, ovarian cancer accounted for only 2.5% of all estimated new cases of female malignancies but accounted for nearly 5% of all deaths due to cancer in women in the United States [2]. Ovarian cancer is not a single disease entity; rather, it is comprised of various histologic subtypes based on differing cells of origin [3]. The most common histologic type of epithelial ovarian cancer is high-grade serous ovarian carcinoma (HGSOC) whereas ovarian clear cell (OCCC), mucinous ovarian (MOC), and low-grade serous ovarian (LGSOC) carcinomas are considered rare epithelial ovarian tumors [4]. The clinical behavior and molecular characteristics of these rare epithelial ovarian tumors are known to be distinct from HGSOC, highlighting the importance of a tailored treatment approach for each tumor subtype [5–7].
As compared to HGSOC, rare epithelial ovarian tumors have been understudied due to their rarity. The past few decades have witnessed various developments in the treatment of ovarian cancer, particularly surgical and chemotherapeutic strategies; however, these have been primarily based on studies of HGSOC in the setting of a clinical trial. A sufficient body of evidence is lacking to describe the population-based trends and statistics for these rare epithelial ovarian tumors [4]. Providing such statistics will be useful for clinicians, researchers, and patients to understand the overview and landscape of rare epithelial ovarian tumors; this will also help to identify salient problems and future directions of treatment and research strategies for these diseases. We describe the evolving population-based statistics for rare epithelial ovarian cancer.
2. Materials and methods
2.1. Data source
This is a retrospective observational study examining the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program. The SEER program is the largest population-based tumor registry in the United States, covering nearly one-third of the U.S. population [8]. Since its inception in 1973, the database spans over four decades and is recognized as a powerful resource for trend and incidence analyses, particularly for rare tumors. Data entry and rigorous quality control for the SEER program are managed by registered and trained personnel [9]. This study was deemed exempt by the University of Southern California Institutional Review Board because of the use of publicly available, deidentified data.
2.2. Eligibility
Women with stage I–IV OCCC, MOC, and LGSOC diagnosed between 1988 and 2016 were included in the analysis. Stage I–IV HGSOCs were also included in the analysis and served as a comparator because this is the most common histologic type of invasive ovarian cancer. Cases were excluded from analysis if histologic types were other than HGSOC, OCCC, MOC, or LGSOC; if the disease was unstaged or of an unknown stage; or if they were diagnosed prior to 1988. Borderline ovarian tumors were not included in the study. Exclusion of older cases was due to a lack of detailed information.
2.3. Clinico-pathological information
Among eligible cases, patient demographics, tumor characteristics, treatment types, and survival outcomes were queried from the SEER database. Patient information included age (<30, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years), year of diagnosis (1988–1993, 1994–1999, 2000–2005, 2006–2011, 2012–2016), race/ethnicity (white, black, Hispanic, Asian, and others), registry area (West, Central, and East), and marital status (single, married, and others).
Tumor characteristics included histologic types as above, tumor differentiation (well, moderate, and poor), lymph node status (number of sampled node and tumor-containing node), and tumor size (<5, 5–9.9, 10–14.9, and ≥15 cm). Treatment types included adnexectomy (yes versus no), hysterectomy (yes versus no), pelvic lymphadenectomy (yes versus no), and chemotherapy (yes versus no/unknown). Survival outcomes included follow-up time and vital status (dead versus alive). Survival information is externally linked with the National Death Index and the state mortality records for verification [10].
2.4. Study definition
Cancer stage was based on the American Joint Committee on Cancer surgical-pathological staging classification [11]. In this study, stage I disease was grouped as early-stage disease whereas stage III–IV diseases were grouped as advanced-stage disease. Histologic subtype was based on the International Classification of Disease for Oncology 3rd edition site/histology validation and the World Health Organization (WHO) histological classification codes as previously described [12,13]. Pelvic lymph node information was based on the database code for Regional Nodes that was introduced in 1988, and lymph node ratio was defined as the ratio of the number of tumor-containing lymph nodes to the total number of harvested lymph nodes.
Among serous ovarian cancer, well-differentiated tumors were grouped as LGSOC whereas moderately- and poorly-differentiated tumors were grouped as HGSOC in a two-tier system as described previously [14]. Overall survival (OS) was defined as the time interval between the ovarian cancer diagnosis and the death from any cause. Patients who were alive were censored at the last follow-up visit. Data without information were grouped as unknown group.
2.5. Statistical approach
For univariable analysis, one-way ANOVA test with post-hoc Bonferroni comparisons, Kruskal-Wallis H test, or chi-square test were used to assess the clinico-pathological demographics across four groups as appropriate. The National Cancer Institute’s Joinpoint Regression Program was used to assess the temporal trends of cancer stage and 5-year OS rate per calendar year [15]. Linear segmented regression with log-transformation was used to assess temporal trends of each segment, and relative change was estimated per the modeled value.
The Kaplan-Meier method was used to construct the survival curves, and OS rates at time point of interest were estimated per each histologic type for descriptive statistics. A Cox proportional hazard regression model was fitted to assess the statistical difference for OS, expressed with hazard ratio (HR) and 95% confidence interval (CI). In this study, pair-wise comparisons were performed to compare each rare tumor type to HGSOC. Stage stratification (early-stage disease versus advanced-stage disease) was further performed.
In each pair-wise comparison between rare tumor histology to HGSOC, propensity score matching was used to corroborate the background differences [16]. A binary logistic regression model was fitted to compute the propensity score in each case. Patient demographics, tumor characteristics, and treatment types were entered in the model. One-to-one propensity score matching between the two groups was performed by utilizing an automated algorithm. The optimal caliper width for estimating differences was equal to 0.2 of the standard deviation for the logit of the propensity score in each pair-wise analysis [17]. Post-matching frequency distribution between the two groups was assessed with standardized difference, and the value of 0.1 or less was considered a good balance [18].
For a sensitivity analysis, time-varying effects of the histologic type on OS were assessed in advanced-stage disease. This is to estimate the impacts of histologic type on OS in the first few years after the ovarian cancer diagnosis, as OCCC and MOC showed distinctively poorer survival compared to HGSOC in a post-hoc assessment. Specifically, each rare tumor type was compared to HGSOC for OS in every one year increment cutoff. In each cutoff year, effects of histology type on OS were examined before the specific cutoff point.
A Two-tailed hypothesis was deployed for all statistical analyses, and a P-value of <0.05 was interpreted as statistically significant. Statistical Package for Social Sciences (SPSS, version 24.0, IBM Corp, Armonk, NY, USA) was used for the analysis. The context of the study was outlined per the STROBE guidelines.
3. Results
3.1. Cohort selection
There were 127,284 cases of ovarian cancer identified during the study period, of which, 113,365 cases had cancer stage information. There were 5780 OCCCs (5.1%, 95% CI 5.0–5.2), 7561 MOCs (6.7%, 95% CI 6.5–6.8), and 2021 LGSOCs (1.8%, 95% CI 1.7–1.9), which represented the rare epithelial ovarian tumors, and compared to 38,199 HGSOCs for analysis (Supplemental Fig. S1).
3.2. Patient demographics
Women with rare tumors were significantly younger compared to HGSOC (mean age: OCCC 56.7, MOC 55.9, LGSOC 55.8, and HGSOC 62.7; P < 0.001) (Table 1). When stratified by stage (Supplemental Tables S1–2), women with MOC were youngest with nearly a decade difference in age compared to those with HGSOC in early-stage disease (mean, 51.2 versus 60.6, P < 0.001). In advanced-stage disease, however, this difference was no longer present (mean, MOC versus HGSOC, 62.4 versus 63.0, P = 0.145). Women with OCCC (30.0%) and MOC (29.9%) were more likely to be non-white compared to those with HGSOC (22.5%, P < 0.001).
Table 1.
Patient demographics per histology type (all stages, N = 53,561).
| Characteristics |
HGSOC |
OCCC |
MOC |
LGSOC |
P-value |
|---|---|---|---|---|---|
| Number | n = 38,199 | n = 5780 | n = 7561 | n = 2021 | |
| Age | 62.7 (±12.3) | 56.7 (±11.9) | 55.9 (±16.7) | 55.8 (±13.5) | <0.001 |
| <30 | 165 (0.4%) | 26 (0.4%) | 520 (6.9%) | 106 (5.2%) | |
| 30–39 | 926 (2.4%) | 330 (5.7%) | 791 (10.5%) | 221 (10.9%) | |
| 40–49 | 4709 (12.3%) | 1272 (22.0%) | 1301 (17.2%) | 394 (19.5%) | |
| 50–59 | 9434 (24.7%) | 1960 (33.9%) | 1815 (24.0%) | 453 (22.4%) | |
| 60–69 | 11,039 (28.9%) | 1345 (23.3%) | 1382 (18.3%) | 417 (20.6%) | |
| 70–79 | 8577 (22.5%) | 620 (10.7%) | 1128 (14.9%) | 290 (14.3%) | |
| ≥80 | 3349 (8.8%) | 227 (3.9%) | 624 (8.3%) | 140 (6.9%) | |
| Year | <0.001 | ||||
| 1988–1993 | 3360 (8.8%) | 487 (8.4%) | 1173 (15.5%) | 289 (14.3%) | |
| 1994–1999 | 4808 (12.6%) | 664 (11.5%) | 1229 (16.3%) | 344 (17.0%) | |
| 2000–2005 | 10,112 (26.5%) | 1470 (25.4%) | 1996 (26.4%) | 599 (29.6%) | |
| 2006–2011 | 10,748 (28.1%) | 1630 (28.2%) | 1673 (22.1%) | 456 (22.6%) | |
| 2012–2016 | 9171 (24.0%) | 1529 (26.5%) | 1490 (19.7%) | 333 (16.5%) | |
| Race/ethnicity | <0.001 | ||||
| White | 29,608 (77.5%) | 4044 (70.0%) | 5297 (70.1%) | 1548 (76.6%) | |
| Black | 2353 (6.2%) | 228 (3.9%) | 614 (8.1%) | 138 (6.8%) | |
| Hispanic | 3542 (9.3%) | 518 (9.0%) | 863 (11.4%) | 228 (11.3%) | |
| Asian | 2219 (5.8%) | 896 (15.5%) | 644 (8.5%) | 82 (4.1%) | |
| Othersa | 477 (1.2%) | 94 (1.6%) | 143 (1.9%) | 25 (1.2%) | |
| Registry | <0.001 | ||||
| West | 21,520 (56.3%) | 3451 (59.7%) | 4049 (53.6%) | 1058 (52.4%) | |
| Central | 7223 (18.9%) | 967 (16.7%) | 1704(22.5%) | 419 (20.7%) | |
| East | 9456 (24.8%) | 1362 (23.6%) | 1808 (23.9%) | 544 (26.9%) | |
| Marital status | <0.001 | ||||
| Single | 5130 (13.4%) | 1345 (23.3%) | 1690 (22.4%) | 397 (19.6%) | |
| Married | 21,008 (55.0%) | 3149 (54.5%) | 3625 (47.9%) | 1099 (54.4%) | |
| Others | 10,915 (28.6%) | 1091 (18.9%) | 1987 (26.3%) | 478 (23.7%) | |
| Unknown | 1146 (3.0%) | 195 (3.4%) | 259 (3.4%) | 47 (2.3%) | |
| Stage | <0.001 | ||||
| I | 3269 (8.6%) | 3293 (57.0%) | 4501 (59.5%) | 687 (34.0%) | |
| II | 3056 (8.0%) | 645 (11.2%) | 492 (6.5%) | 219 (10.8%) | |
| III | 20,916 (54.8%) | 1237 (21.4%) | 1395 (18.4%) | 861 (42.6%) | |
| IV | 10,958 (28.7%) | 605 (10.5%) | 1173 (15.5%) | 254 (12.6%) | |
| Nodal metastasisa | <0.001 | ||||
| No | 9114 (51.0%) | 3228 (84.6%) | 3255 (90.7%) | 713 (68.6%) | |
| Yes | 8733 (48.9%) | 603 (15.3%) | 330 (9.2%) | 325 (31.3%) | |
| Unknown | 19 (0.1%) | 5 (0.1%) | 2 (0.1%) | 1 (0.1%) | |
| LNR | 50.0 (IQR 18.2–100) | 33.3 (IQR 12.5–69.0) | 33.3 (IQR 12.5–100) | 28.6 (IQR 14.3–66.7) | <0.001 |
| Tumor size (cm) | <0.001 | ||||
| <5 | 6154 (16.1%) | 723 (12.5%) | 932 (12.3%) | 386 (19.1%) | |
| 5–9.9 | 8584 (22.5%) | 1064 (18.4%) | 703 (9.3%) | 406 (20.1%) | |
| 10–14.9 | 5487 (14.4%) | 1220 (21.1%) | 821 (10.9%) | 228 (11.3%) | |
| ≥15 | 3195 (8.4%) | 1380 (23.9%) | 2145 (28.4%) | 203 (10.0%) | |
| Unknown | 14,779 (38.7%) | 1393 (24.1%) | 2960 (39.1%) | 798 (39.5%) | |
| Oophorectomy | <0.001 | ||||
| No | 1718 (4.5%) | 167 (2.9%) | 762 (10.1%) | 65 (3.2%) | |
| Yes | 36,227 (94.8%) | 5592 (96.7%) | 6731 (89.0%) | 1945 (96.2%) | |
| NOSa | 254 (0.7%) | 21 (0.4%) | 68 (0.9%) | 11 (0.5%) | |
| Hysterectomy | <0.001 | ||||
| No | 5271 (13.8%) | 702 (12.1%) | 2302 (30.4%) | 383 (19.0%) | |
| Yes | 29,708 (77.8%) | 4813 (83.3%) | 4745 (62.8%) | 1463 (72.4%) | |
| Unknown | 3220 (8.4%) | 265 (4.6%) | 514 (6.8%) | 175 (8.7%) | |
| Lymphadenectomy | <0.001 | ||||
| No | 20,112 (52.7%) | 1822 (31.5%) | 3910 (51.7%) | 971 (48.0%) | |
| Yes | 17,866 (46.8%) | 3936 (68.1%) | 3587 (47.4%) | 1039 (51.4%) | |
| Unknown | 221 (0.6%) | 22 (0.4%) | 64 (0.8%) | 11 (0.5%) | |
| Sampled nodesb | 9 (IQR 3–19) | 12 (IQR 5–21) | 10 (IQR 4–18) | 10 (IQR 4–19) | <0.001 |
| Chemotherapy | <0.001 | ||||
| No | 7349 (19.2%) | 1590 (27.5%) | 4388 (58.0%) | 835 (41.3%) | |
| Yes | 30,850 (80.8%) | 4190 (72.5%) | 3173 (42.0%) | 1186 (58.7%) |
Number (percentage per column), mean (±standard deviation), or median (interquartile range) is shown. One-way ANOVA test, Kruskal-Wallis H test, or chi-square test for P-values. Abbreviations: IQR, interquartile range; NOS, not otherwise specified; HGSOC, high-grade serous ovarian carcinoma; OCCC, ovarian clear cell carcinoma; MOC, mucinous ovarian carcinoma; and LGSOC, low-grade serous ovarian carcinoma.
Including unknown.
Among staged cases
In early-stage disease, chemotherapy was commonly used for HGSOC (61.0%) and OCCC (68.3%) but less commonly in MOC (27.9%) and LGSOC (28.1%) (P < 0.001). Women with early-stage OCCC were more likely to undergo lymphadenectomy compared to other histologic types (74.8% versus 52.5–63.3%, P < 0.001). In advanced-stage disease, women with MOC were least likely to receive chemotherapy compared to other histologic types (63.2% versus 77.1–83.2%, P < 0.001).
3.3. Stage characteristics
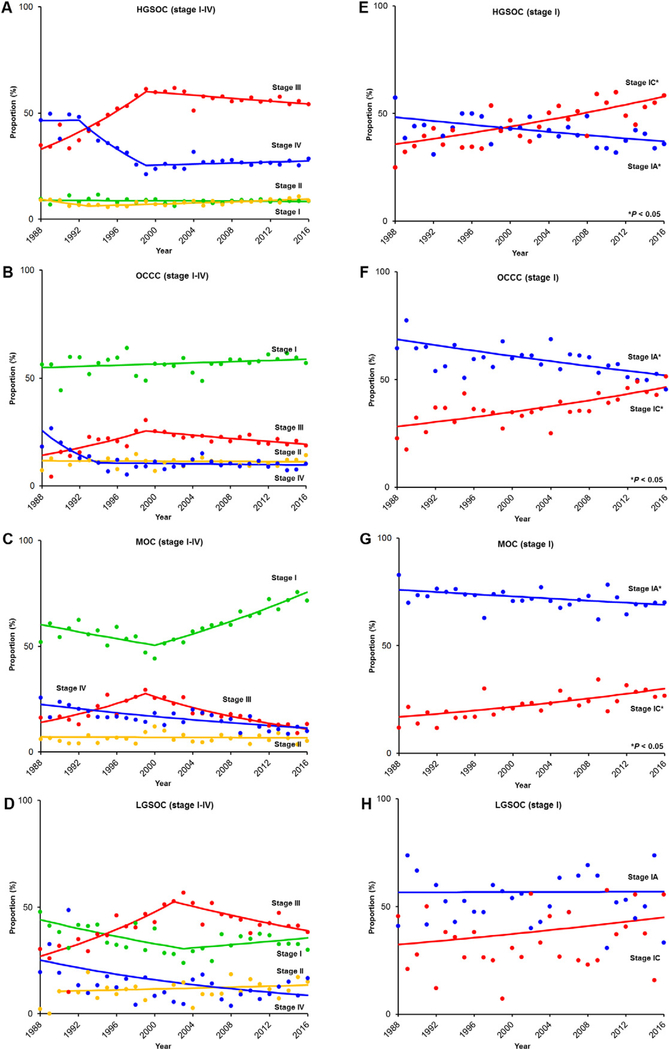
Rare tumors, particularly OCCC and MOC, were more likely to be stage I compared to HGSOC: 57.0% for OCCC, 59.5% for MOC, 34.0% for LGSOC, and 8.6% for HGSOC (P < 0.001; Table 1 and Fig. 1A–D).The number of stage I MOC has increased (50.5% to 75.6%, 1.5-fold increase) from 2000 to 2016 whereas the number of stage III–IV tumors has decreased in recent years (stage III, 27.6% to 11.0%, 60.3% relative decrease from 1999 to 2016; and stage IV, 22.6% to 11.4%, 49.6% relative decrease from 1988 to 2016) (all, P < 0.001). Among stage I cases, (Fig. 1E–H), there was a stage-shift from stage IA to IC disease for HGSOC, OCCC, and MOC, with a significant increase in stage IC disease for all three of these tumor types (all, P < 0.05).
Fig. 1.
Temporal trends of stage shift per histologic type. Temporal trend of cancer stage is shown per histology type for stage I–IV diseases (HGSOC for panel A, OCCC for panel B, MOC for panel C, and LGSOC for panel D) and stage I sub-stage disease diseases (HGSOC for panel E, OCCC for panel F, MOC for panel G, and LGSOC for panel H). Abbreviations: OCCC, ovarian clear cell carcinoma; MOC, mucinous ovarian cancer; LGSOC, low-grade serous ovarian cancer; and HGSOC, high-grade serous ovarian cancer.
3.4. Population-level survival trends
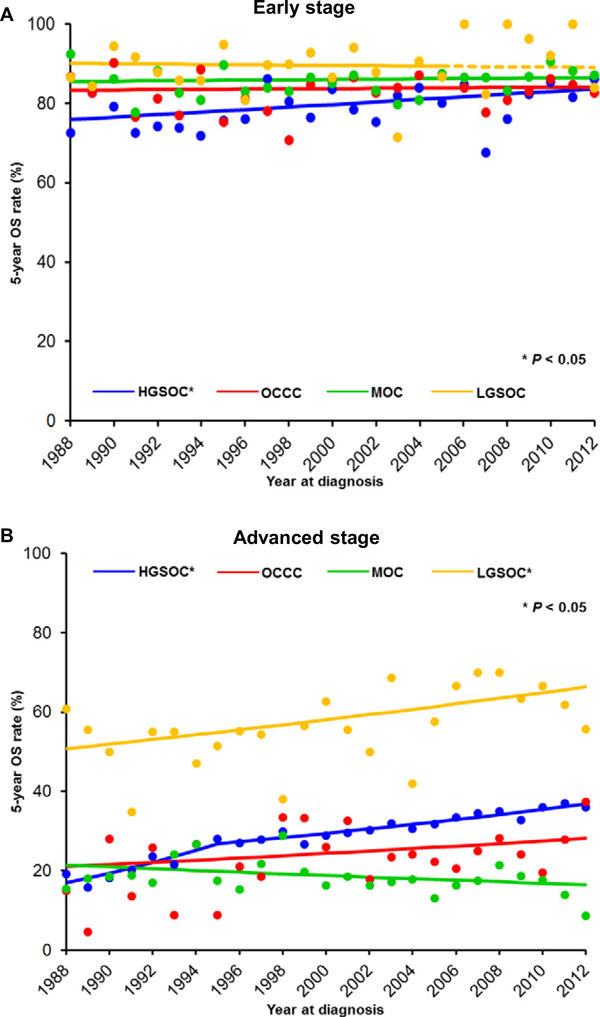
For the early-stage cohort (n = 11,750), the median follow-up of for censored cases was 7.9 (IQR 3.3–14.3) years, and 3455 (29.4%) deaths occurred during the follow-up. The 5-year OS rates have increased significantly for HGSOC (75.9% to 83.7%, 10.3% relative increase, P = 0.028; Fig. 2A); but the 5-year OS rates have remained unchanged in all three of the rare tumor types (all, P > 0.05).
Fig. 2.
The 5-year overall survival rates per histologic type. 5-year OS rates per histology types are shown for A) early-stage disease and B) advanced-stage disease. Dashed line in panel A indicated the estimated value based on 1988–2005 value. Abbreviations: OCCC, ovarian clear cell carcinoma; MOC, mucinous ovarian cancer; LGSOC, low-grade serous ovarian cancer; and HGSOC, high-grade serous ovarian cancer. Dots indicated observed value and lines indicated modeled values.
For the advanced-stage cohort (n = 37,399), the median follow-up of censored cases was 3.7 (IQR 1.5–8.3) years, and there were 27,651 (73.9%) deaths observed during the follow-up period. The 5-year OS rates have significantly increased in HGSOC (16.9% to 36.8%, 2.2-fold increase, P < 0.001) and LGSOC (50.8% to 66.4%, 30.7% relative increase, P = 0.010) but remain unchanged in OCCC (21.0% to 28.2%, P = 0.174) and MOC (21.4% to 16.5%, P = 0.102; Fig. 2B).
3.5. Patient-level survival outcome
Pair-wise comparisons to the HGSOC group were performed for each rare tumor group, respectively, and all the covariates were well-balanced between the two groups after propensity score matching (all, standardized difference ≤ 0.10; Supplemental Table S3–8).
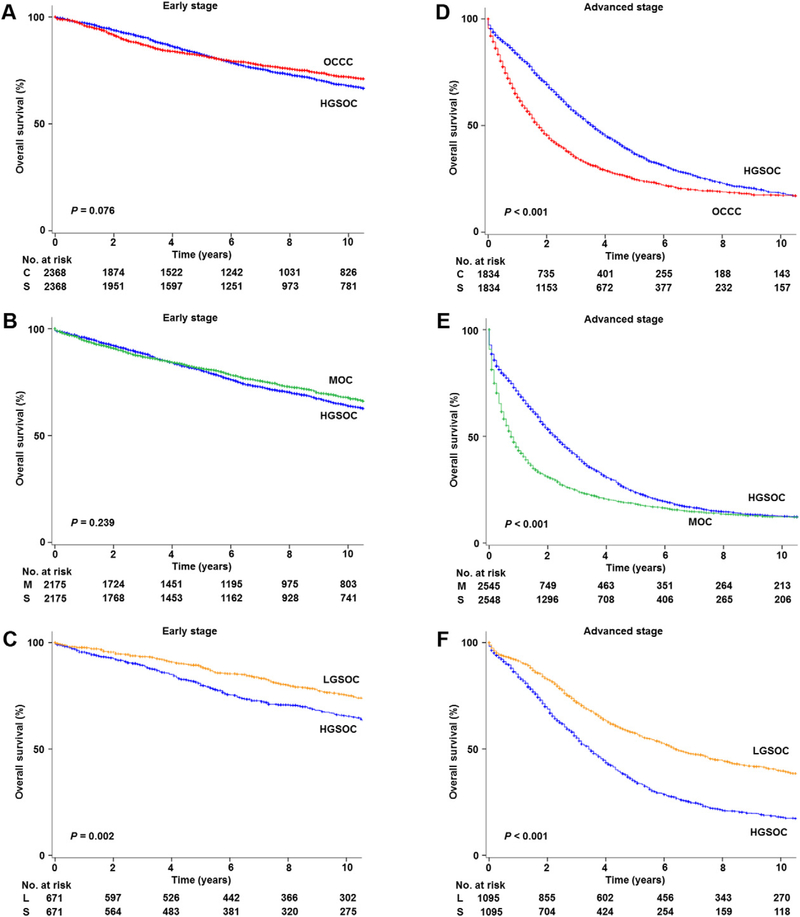
For early-stage disease (Fig. 3A–C and Table 2), women with OCCC (8-year rates, 75.8% versus 73.2%, 2.6% absolute difference, HR 0.91, 95% CI 0.82–1.01, P = 0.076) and MOC (72.8% versus 70.3%, 2.5% absolute difference, HR 0.94, 95% CI 0.85–1.04, P = 0.239) had similar OS to HGSOC whereas LGSOC had significantly better OS (80.1% versus 70.7%, 9.4% absolute difference, HR 0.93, 95% CI 0.89–0.97, P = 0.002) compared to those with HGSOC.
Fig. 3.
Stage-specific overall survival based on histologic type (propensity score matched). Log-rank test for P-values. Abbreviations: OCCC, ovarian clear cell carcinoma; MOC, mucinous ovarian cancer; LGSOC, low-grade serous ovarian cancer; and HGSOC, high-grade serous ovarian cancer.
Table 2.
Stage-specific overall survival per histology type (propensity score matched).
| Stage | Time | OCCC vs HGSOC | Survival difference | MOC vs HGSOC | Survival difference | LGSOC vs HGSOC | Survival difference |
|---|---|---|---|---|---|---|---|
| Early | 2-year | 91.9% vs 94.0% | −2.1% | 91.0% vs 92.2% | −1.2% | 95.5% vs 92.8% | 2.7% |
| 3-year | 87.2% vs 90.7% | −3.5% | 87.2% vs 88.7% | −1.5% | 93.4% vs 89.5% | 3.9% | |
| 5-year | 82.0% vs 82.7% | −0.7% | 81.5% vs 80.8% | 0.7% | 89.3% vs 80.8% | 8.5% | |
| 8-year | 75.8% vs 73.2% | 2.6% | 72.8% vs 70.3% | 2.5% | 80.1% vs 70.7% | 9.4% | |
| 10-year | 71.9% vs 67.9% | 4.0% | 67.9% vs 64.2% | 3.7% | 75.4% vs 65.6% | 9.8% | |
| Advanced | 2-year | 46.4% vs 70.5% | −24.1% | 31.4% vs 54.2% | −22.8% | 83.2% vs 70.0% | 13.2% |
| 3-year | 35.7% vs 56.9% | −21.2% | 24.9% vs 41.5% | −16.6% | 72.6% vs 56.2% | 16.4% | |
| 5-year | 25.2% vs 37.5% | −12.3% | 18.4% vs 24.0% | −5.6% | 57.7% vs 35.3% | 22.4% | |
| 8-year | 19.0% vs 23.2% | −4.2% | 13.6% vs 14.7% | −1.1% | 44.8% vs 21.5% | 23.3% | |
| 10-year | 17.3% vs 18.4% | −1.1% | 12.2% vs 12.6% | −0.4% | 39.8% vs 18.0% | 21.8% |
Overall survival rates are shown at each time point. Survival statistics are derived from propensity score matched model in each comparison. Survival difference indicated relative survival rate difference to HGSOC at each time point. Abbreviations: HGSOC, high-grade serous ovarian carcinoma; OCCC, ovarian clear cell carcinoma; MOC, mucinous ovarian carcinoma; and LGSOC, low-grade serous ovarian carcinoma.
Conversely, for advanced-stage disease (Fig. 3D–F and Table 2), OCCC (5-year rates, 25.2% versus 37.5%, 12.3% absolute difference, HR 1.42,95% CI 1.32–1.53, P < 0.001) and MOC (18.4% versus 24.0%, 5.6% absolute difference, HR 1.11, 95% CI 1.09–1.13, P < 0.001) had significantly poorer OS whereas LGSOC (57.7% versus 35.3%, 22.4% absolute difference, HR 0.86, 95% CI 0.84–0.89, P < 0.001) had superior OS compared to HGSOC.
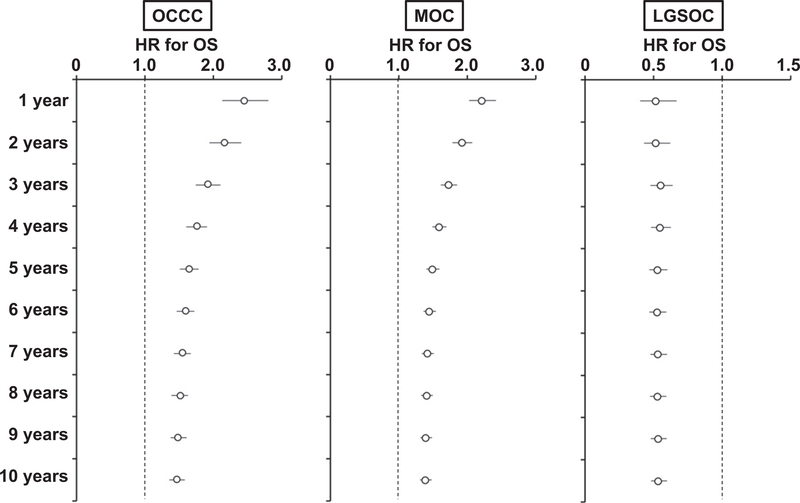
As there was a large absolute difference for OS in the first few years following diagnosis between advanced-stage HGSOC and advanced-stage OCCC (21.2–24.1% inferior to HGSOC) as well as advanced-stage MOC (16.6–22.8% inferior to HGSOC) (Table 2), time-varying effects of these histologic types on survival estimates for OS were assessed in advanced-stage disease (Fig. 4). The analysis demonstrated that OCCC (HR range, 1.92–2.45) and MOC (HR range, 1.73–2.22) had particularly poorer OS in the first three years following diagnosis compared to HGSOC.
Fig. 4.
Time-varying effects of histology on overall survival in advanced-stage disease. Time varying effects of histology type on OS were shown before and after the cutoff time point. Empty circles represent adjusted-HR and bars represent 95% confidence interval. Abbreviations: HR, hazard ratio; OS, overall survival; MOC, mucinous ovarian carcinoma; and HGSOC, high-grade serous ovarian carcinoma.
4. Discussion
Key findings of the study are that (i) rare epithelial ovarian tumors account for 2–7% of epithelial ovarian cancer, (ii) patient and tumor characteristics are different across these rare tumors, and (iii) advanced-stage OCCC and MOC have distinctively poor outcomes in the first few years from diagnosis. Several areas warrant further discussion.
4.1. OCCC
Unlike the Japanese population, where OCCC is one of the dominant histological subtypes, reaching nearly 30% in recent years [19], OCCC was seen in approximately 5% in our study, representing a rare tumor in the United States. Salient findings in this disease are that the affected women are typically young and have early-disease which carries a generally good prognosis. Once the disease becomes metastatic, the prognosis is considerably worse than HGSOC.
Our study validates the results of recent large-scale studies demonstrating poor OS in advanced-stage OCCC, particularly in the first few years following diagnosis, as well as comparable to superior OS in early-stage OCCC when compared to HGSOC [20,21]. Our study, additionally, provides specific information, including an absolute difference for OS exceeding 20% for OCCC compared to advanced-stage HGSOC over this same time period. The larger sample size of OCCC in our study compared to the prior study adds significant weight to their conclusions (SEER versus GOG: 1842 versus 275 cases) [20].
Limited effectiveness of current chemotherapy regimens for OCCC likely contributes to the poor clinical outcomes [22,23]. Various cytotoxic and biological agents have been previously tested for OCCC but have shown minimal to modest effectiveness in this disease [7]. Currently, trials examining the effectiveness of immunotherapy for the treatment of OCCC are ongoing and may provide a new approach for improving outcomes for patients with advanced disease [7]. Another possible explanation for the poor survival in advanced-stage OCCC may be aggressive tumor biology (e.g., increased interleukin 6 levels, which are highly associated with the characteristic high rate of venous thromboembolism in advanced-stage OCCC) [7,21]. Thus, integrating biology-driven tumor targeting may be useful in the treatment of advanced-stage OCCC.
4.2. MOC
Similar to OCCC, MOCs have traditionally been thought to occur in young women; however, our study demonstrated that this finding only applies to those with early-stage disease. Remarkably, a stage-shift has occurred from advanced- to early-stage disease in MOC in recent years. The causality of this observation is not entirely clear but may be due to improved awareness and recognition of the spectrum of mucinous tumor types. This includes identification of metastatic MOC versus metastatic tumors from other origins such as the gastrointestinal (GI) tract, as well as discrimination of MOC from mucinous borderline ovarian tumors [24,25]. Another possible cause may be earlier disease detection. In a stage-specific analysis (Fig. 1C), the number of cases of stage III disease started decreasing around the early-2000s. This is around the same time in which there was a push for public awareness of ovarian cancer symptoms [26,27], and it will be of interest to further investigate if this may have contributed to this stage-shift. Supporting this speculation is the decreasing number of cases with stage III disease, which seems to be universal across all of the histology subtypes.
Prior studies have shown that early-stage MOC has comparable to superior survival compared to HGSOC whereas advanced-stage MOC carries a worse prognosis [28–33]. As these prior studies have been relatively limited in sample size and follow-up, inclusion of nearly 7500 cases with prolonged follow-up in our study markedly enhances the interpretation of the results. Our study adds new information in that survival of women with advanced-stage MOC is strikingly dismal in the first few years following diagnosis compared to those with advanced-stage HGSOC.
As platinum-based doublets remain the mainstay of treatment of advanced-stage epithelial ovarian cancer, the poor prognosis in advanced-stage MOC may be secondary to the known platinum-resistance of these tumors [28,34]. Due to the biological similarity of MOC to mucinous tumors of GI tract origin, multiple guidelines have incorporated GI-based chemotherapeutic approaches in the management of MOC [35,36]. Yet, the effectiveness of this approach remains unknown due to early closure of a recent GOG trial [37]. As women with advanced-stage MOC were least likely to have received chemotherapy, this might also have contributed to the poor outcomes. It is unknown if the low rate of chemotherapy use in advanced-stage MOC is due to patient factors (e.g., older age, comorbidity, and performance status) or physician choice, and further studies are warranted.
Our study suggests that early-stage and advanced-stage MOCs may possess different tumor characteristics and biology, with indolent activity in early-stage disease versus aggressive behavior in the advanced-stage counterpart. Histologic architectural patterns may be a plausible link to these findings. The expansile (confluent) subtype of MOC represents an indolent tumor and can correspond to early-stage disease whereas the infiltrative subtype represents an aggressive tumor and can correspond to advanced-stage disease [5]. Tumor classification based on this histologic architecture pattern was recently incorporated into the 2014 WHO system, and practical utility in the management of MOC based on this change is expected [5].
4.3. LGSOC
LGSOC is a relatively new classification that was proposed in the mid-1980s [38], and is the least common type of rare epithelial ovarian tumors among those examined in our population-based analysis. LGSOC is likely the most understudied of these three rare tumors. There has been a decreasing number of diagnosed cases of LGSOC in recent years [39], and the number of LGSOC shown in our study was lower than what was reported in the past as 6–10% [40]. It is speculated that this decreasing trend of LGSOC in recent years may be due to proper diagnosis of LGSOC over serous borderline ovarian tumors [39]. Collectively, these results highlight the importance of centralizing care, optimizing diagnostic processes, and continuing research for this disease.
The prognosis of women with LGSOC was superior to that of HGSOC for both early- and advanced-stage tumors. Moreover, population-level survival has improved significantly for advanced-stage LGSOC over time. The exact reason for this improvement in survival is unknown, but the use of hormonal therapy and anti-angiogenic agents may be a contributing factor and warrants prospective study to confirm the effectiveness of these strategic approaches in LGSOC [41,42]. Population-level survival has also improved significantly for HGSOC from 1988 to 2016. This trend was observed in both early- and advanced-stage disease. This finding is consistent with a recent population-based analysis that reported an improved relative survival in ovarian cancer from 1975 to 2011 [43]. As their study did not examine the histology-specific trend, our study provides more information with regard to the improved survival seen in serous tumors.
4.4. Strengths and limitations
Strengths of the study include that this is a population-based study utilizing the largest tumor registry in the United States. Propensity score matching enhanced the statistical rigor that is particularly useful as background characteristics differ across the tumor types. However, there are several limitations in this study. First, the SEER program covers only a fraction of the U.S. population, and may exclude unique geographic areas with relatively distinct populations. Thus, it is unknown if the statistics of in this study reflect the entire U.S. population or instead are due to a possible selection bias, and would not be reproducible in different populations (generalizability).
Second, the SEER program does not have information for disease recurrence, and therefore, complete oncological outcome analysis was not feasible in this study. Third, chemotherapy plays a pivotal role in the management of epithelial ovarian cancer but regimen type, accuracy, and toxicity of chemotherapy was not available in this study. Fourth, more detailed tumor information such as tumor markers, molecular testing, and genetic alterations were not available in the SEER program but these tests have been incorporated into treatment algorithms in recent guidelines [36].
Last, and perhaps most importantly, central pathology review was not available in this database. Therefore, the accuracy of histological diagnoses in this study is unknown. This is particularly important for MOC as considerable numbers of MOC are found on review by expert gynecologic pathologists to have originated from somewhere other than the ovary [5]. Moreover, the diagnosis of LGSOC is based on the composition of tumor differentiation and histology type but not based on central pathology review.
4.5. Summary
Clearly, the prognosis for women with HGSOC has significantly improved as a population in the past several decades. This is particularly pronounced in advanced-stage disease where the population-level survival has more than doubled. Similarly, population-level survival has improved in advanced-stage LGSOC. In contrast, the population-level survival in advanced-stage OCCC and MOC has not improved, exhibiting disproportionally poor survival in these diseases compared to advanced-stage HGSOC. Our study team endorses this as a major problem, highlighting the need for further research into novel treatment strategies. Centralization of patient care and national/international collaborative work are the keys to improve the outcome for rare epithelial ovarian cancer.
Supplementary Material
HIGHLIGHTS.
Populational characteristics and outcomes of rare ovarian tumor were examined
OCCC, MOC, and LGSOC have distinct clinico-pathological characteristics compared to HGSOC
OCCC versus HGSOC: comparable survival in early disease while dismal survival in advanced disease
MOC versus HGSOC: comparable survival in early disease while dismal survival in advanced disease
LGSOC versus HGSOC: superior survival in both early and advanced disease
Acknowledgments
Funding support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Declaration of competing interest
Consultant, Clovis Oncology, Tesaro, research funding, Merck (J.D.W.); scientific consulting, Kiyatec, Merck, shareholder, Biopath, research funding, M-Trap (A.K.S.); Stock and other ownership interests, Celgene, Johnson & Johnson, Biogin, consulting or advisory role, Clovis Oncology, research funding, Novartis, royalties from Elsevier as book editor, royalties from UpToDate for authorship (D.M.G.); honorarium, Chugai, textbook editorial expense, Springer, investigator meeting attendance expense, VBL therapeutics (K.M.); consultant, Quantgene (L.D.R); research funding, MSD (none for others).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.11.122.
References
- [1].Coleman RL, Liu JS, Matsuo K, Thaker PH, Westin SN, Sood AK, Carcinoma of the ovaries and fallopian tubes, in: Niederhuber JE, Armitage AO, Doroshow JH, Kastan MB, Tepper JE (Eds.), Abeloff’s Clinical Oncology, sixth editionElsevier, Philadelphia: 2019, pp. 1525–1543. [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA Cancer J. Clin 69 (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [3].Vaughan S, Coward JI, Bast RC Jr., Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK, Stronach EA, Walczak H, Bowtell DD, Balkwill FR, Rethinking ovarian cancer: recommendations for improving outcomes, Nat. Rev. Cancer 11 (2011) 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lheureux S, Gourley C, Vergote I, Oza AM, Epithelial ovarian cancer, Lancet 393 (2019) 1240–1253. [DOI] [PubMed] [Google Scholar]
- [5].Morice P, Gouy S, Leary A, Mucinous ovarian carcinoma, N. Engl. J. Med 380 (2019) 1256–1266. [DOI] [PubMed] [Google Scholar]
- [6].Gershenson DM, Low-grade serous carcinoma of the ovary or peritoneum, Ann. Oncol. 27 (2016) i45–i49. [DOI] [PubMed] [Google Scholar]
- [7].Oda K, Hamanishi J, Matsuo K, Hasegawa K, Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management, Gynecol. Oncol. 151 (2018) 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].The surveillance, epidemiology, and end results (SEER) program of the National Cancer Institute, https://seer.cancer.gov/, Accessed date: 30 July 2019.
- [9].National Cancer Registrars Association, http://www.ncra-usa.org, Accessed date: 30 July 2019.
- [10].National death index, Centers for Disease Control and Prevention https://www.cdc.gov/nchs/ndi/index.htm, Accessed date: 30 July 2019.
- [11].American Joint Committee on Cancer, https://cancerstaging.org/Pages/default.aspx, Accessed date: 30 July 2019.
- [12].Matsuo K, Machida H, Stone RL, Soliman PT, Thaker PH, Roman LD, Wright JD, Risk of subsequent ovarian cancer after ovarian conservation in young women with stage I endometrioid endometrial cancer, Obstet. Gynecol 130 (2017) 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Matsuo K, Machida H, Horowitz MP, Shahzad MMK, Guntupalli SR, Roman LD, Wright JD, Risk of metachronous ovarian cancer after ovarian conservation in young women with stage I cervical cancer, Am. J. Obstet. Gynecol. 217 (2017) 580 e1–580 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bodurka DC, Deavers MT, Tian C, Sun CC, Malpica A, Coleman RL, Lu KH, Sood AK, Birrer MJ, Ozols R, Baergen R, Emerson RE, Steinhoff M, Behmaram B, Rasty G, Gershenson DM, Reclassification of serous ovarian carcinoma by a 2-tier system: a gynecologic oncology group study, Cancer 118 (2012) 3087–3094. [DOI] [PubMed] [Google Scholar]
- [15].Kim HJ, Fay MP, Feuer EJ, Midthune DN , Permutation tests for joinpoint regression with applications to cancer rates, Stat. Med 19 (2000) 335–351. [DOI] [PubMed] [Google Scholar]
- [16].Hershman DL, Wright JD, Comparative effectiveness research in oncology methodology: observational data, J. Clin. Oncol 30 (2012) 4215–4222. [DOI] [PubMed] [Google Scholar]
- [17].Austin PC, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies, Pharm. Stat.10 (2011) 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, Berry MF, Schrag D, Pang HH, Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies, J. Natl. Cancer Inst. 109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Machida H, Matsuo K, Yamagami W, Ebina Y, Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T, Mikami M, Trends and characteristics of epithelial ovarian cancer in Japan between 2002 and 2015: a JSGO-JSOG joint study, Gynecol. Oncol. 153 (2019) 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Oliver KE, Brady WE, Birrer M, Gershenson DM, Fleming G, Copeland LJ, Tewari K, Argenta PA, Mannel RS, Secord AA, Stephan JM, Mutch DG, Stehman FB, Muggia FM, Rose PG, Armstrong DK, Bookman MA, Burger RA, Farley JH, An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: an NRG oncology/gynecologic oncology group experience, Gynecol. Oncol. 147 (2017) 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matsuo K, Hasegawa K, Yoshino K, Murakami R, Hisamatsu T, Stone RL, Previs RA, Hansen JM, Ikeda Y, Miyara A, Hiramatsu K, Enomoto T, Fujiwara K, Matsumura N, Konishi I, Roman LD, Gabra H, Fotopoulou C, Sood AK, Venous thromboembolism, interleukin-6 and survival outcomes in patients with advanced ovarian clear cell carcinoma, Eur. J. Cancer 51 (2015) 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K, Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy, Cancer 88 (2000) 2584–2589. [PubMed] [Google Scholar]
- [23].Mabuchi S, Sugiyama T, Kimura T, Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives, J. Gynecol. Oncol. 27 (2016) e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matsuo K, Machida H, Mandelbaum RS, Grubbs BH, Roman LD, Sood AK, Gershenson DM, Mucinous borderline ovarian tumor versus invasive well-differentiated mucinous ovarian cancer: difference in characteristics and outcomes, Gynecol. Oncol. 153 (2019) 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Perren TJ, Mucinous epithelial ovarian carcinoma, Ann. Oncol. 27 (2016) i53–i57. [DOI] [PubMed] [Google Scholar]
- [26].Goff BA, Mandel L, Muntz HG, Melancon CH, Ovarian carcinoma diagnosis, Cancer 89 (2000) 2068–2075. [DOI] [PubMed] [Google Scholar]
- [27].Goff BA, Mandel LS, Melancon CH, Muntz HG, Frequency of symptoms of ovarian cancer in women presenting to primary care clinics, JAMA 291 (2004) 2705–2712. [DOI] [PubMed] [Google Scholar]
- [28].Frumovitz M, Schmeler KM, Malpica A, Sood AK, Gershenson DM, Unmasking the complexities of mucinous ovarian carcinoma, Gynecol. Oncol. 117 (2010) 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Crane EK, Brown J, Early stage mucinous ovarian cancer: a review, Gynecol. Oncol. 149 (2018) 598–604. [DOI] [PubMed] [Google Scholar]
- [30].Matsuo K, Nishimura M, Bottsford-Miller JN, Huang J, Komurov K, Armaiz-Pena GN, Shahzad MM, Stone RL, Roh JW, Sanguino AM, Lu C, Im DD, Rosenshien NB, Sakakibara A, Nagano T, Yamasaki M, Enomoto T, Kimura T, Ram PT, Schmeler KM, Gallick GE, Wong KK, Frumovitz M, Sood AK, Targeting SRC in mucinous ovarian carcinoma, Clin. Cancer Res. 17 (2011) 5367–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Winter WE 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP, Prognostic factors for stage III epithelial ovarian cancer: a gynecologic oncology group study, J. Clin. Oncol 25 (2007) 3621–3627. [DOI] [PubMed] [Google Scholar]
- [32].Hess V, A’Hern R, Nasiri N, King DM, Blake PR, Barton DP, Shepherd JH, Ind T, Bridges J, Harrington K, Kaye SB, Gore ME, Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment, J. Clin. Oncol. 22 (2004) 1040–1044. [DOI] [PubMed] [Google Scholar]
- [33].Schiavone MB, Herzog TJ, Lewin SN, Deutsch I, Sun X, Burke WM, Wright JD, Natural history and outcome of mucinous carcinoma of the ovary, Am. J. Obstet. Gynecol. 205 (2011) 480 e1–8. [DOI] [PubMed] [Google Scholar]
- [34].Shimada M, Kigawa J, Ohishi Y, Yasuda M, Suzuki M, Hiura M, Nishimura R, Tabata T, Sugiyama T, Kaku T, Clinicopathological characteristics of mucinous adenocarcinoma of the ovary, Gynecol. Oncol. 113 (2009) 331–334. [DOI] [PubMed] [Google Scholar]
- [35].Ledermann JA, Luvero D, Shafer A, O’Connor D, Mangili G, Friedlander M, Pfisterer J, Mirza MR, Kim JW, Alexandre J, Oza A, Brown J, Gynecologic Cancer InterGroup (GCIG) consensus review for mucinous ovarian carcinoma, Int. J. Gynecol. Cancer 24 (2014) S14–S19. [DOI] [PubMed] [Google Scholar]
- [36].Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, NCCN Clinical Practice Guidelines in Oncology http://www.nccn.org/patients, Accessed date: 12 August 2019.
- [37].Gore M, Hackshaw A, Brady WE, Penson RT, Zaino R, McCluggage WG, Ganesan R, Wilkinson N, Perren T, Montes A, Summers J, Lord R, Dark G, Rustin G, Mackean M, Reed N, Kehoe S, Frumovitz M, Christensen H, Feeney A, Ledermann J, Gershenson DM, An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor, Gynecol. Oncol. 153 (2019) 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bell DA, Low-grade serous tumors of ovary, Int. J. Gynecol. Pathol. 33 (2014) 348–356. [DOI] [PubMed] [Google Scholar]
- [39].Matsuo K, Machida H, Grubbs BH, Sood AK, Gershenson DM, Trends of low-grade serous ovarian carcinoma in the United States, J. Gynecol. Oncol. 29 (2018) e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Romero I, Sun CC, Wong KK, Bast RC Jr., D.M. Gershenson, Low-grade serous carcinoma: new concepts and emerging therapies, Gynecol. Oncol. 130 (2013) 660–666. [DOI] [PubMed] [Google Scholar]
- [41].Gershenson DM, Bodurka DC, Coleman RL, Lu KH, Malpica A, Sun CC, Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum, J. Clin. Oncol 35 (2017) 1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dalton HJ, Fleming ND, Sun CC, Bhosale P, Schmeler KM, Gershenson DM, Activity of bevacizumab-containing regimens in recurrent low-grade serous ovarian or peritoneal cancer: a single institution experience, Gynecol. Oncol. 145 (2017) 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wright JD, Chen L, Tergas AI, Patankar S, Burke WM, Hou JY, Neugut AI, Ananth CV, Hershman DL, Trends in relative survival for ovarian cancer from 1975 to 2011, Obstet. Gynecol. 125 (2015) 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.