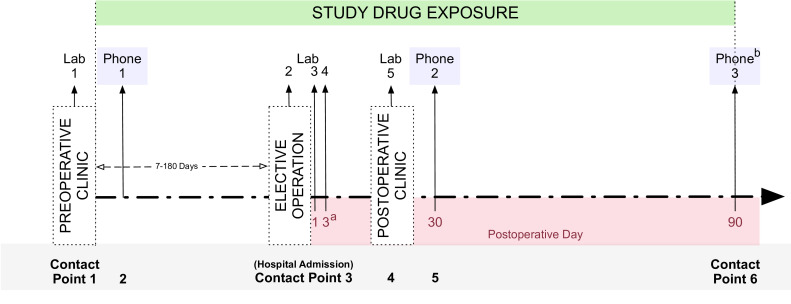
Figure 3.
SPRY-Metformin timeline. aIf patients are discharged on the day of the surgical intervention, laboratory sample 4 will be omitted. If hospital discharge occurs prior to postoperative day 3, laboratory sample 4 occur immediately prior to discharge. bLongitudinal testing at contact point 6 testing is dependent on participation in the motor subgroup (table 1). Patients are recruited, consented by providers, randomised, undergo baseline venous blood sampling and are provided study drug at preoperative clinic (contact point 1). In the 7–180 preoperative days, patients undergo baseline testing (table 1), and both patient safety and study drug compliance are monitored via phone interview (contact point 2). Three venous blood samples are coupled with clinical blood draws throughout the operative hospital admission (contact point 3). A final venous sample is collected in standard of care postoperative clinic (contact point 4). At postoperative days 30 and 90, patients are contacted to monitor both patient safety and study drug compliance, collect postoperative outcomes (box 2) and complete additional outcome testing (table 1).