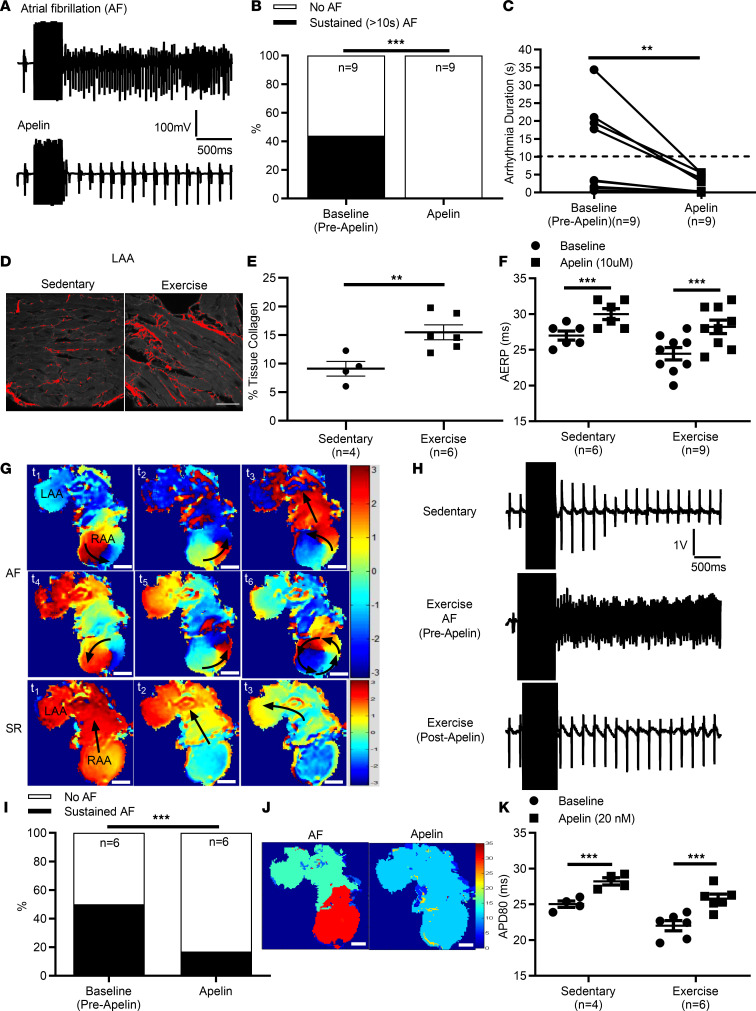
Figure 2. Direct in vivo and ex vivo effects of PYRapelin13 on atrial arrhythmia inducibility on swim-trained mice.
Vulnerability to atrial arrhythmias was determined in vivo using an intracardiac octapolar catheter placed in the right ventricle, followed by programmed electrical stimulations. (A) Representative intracardiac bipolar electrograms showing inducibility of atrial fibrillation (AF) before and following PYRapelin13 (10 μM i.p.) injection in exercised mice. (B) The percentage of exercised mice with sustained (>10 s) AF (44% vs. 0%, P < 0.0001) as well as arrhythmia durations (P = 0.0039) decreased (C) following PYRapelin13 administration. (D and E) Intense exercise caused elevated (P = 0.0103) atrial fibrosis compared with sedentary mice. (F) Decreased arrhythmia vulnerability with PYRapelin13 was associated with prolongation (~16%, P = 0.0004) of the atrial effective refractory period (AERP), which was also observed in sedentary control mice (~11%). (G) Vulnerability to arrhythmias was determined ex vivo in isolated (denervated) atrial preparations from swim-trained (n = 6) and sedentary control (n = 4) mice. Representative field recordings showing the baseline and inducibility of atrial arrhythmias before and following PYRapelin13 (20 nM) administration. (H) A typical representative AF event initiated in the right atrial appendage (RAA) by programmed electrical stimulation recorded by optical mapping of atria loaded with Di-4-ANEPPS showing rotor formation in the RAA, characterized by rapid focal activity, reentry circuits, and 2:1 conduction block. AF inducibility was decreased (P < 0.0001) following PYRapelin13 administration, with normal conduction observed with 90 ms pacing at the RAA. (I) The percentage of exercised mice with sustained (>10 s) atrial arrhythmias decreased (50% to 17%, P < 0.0001) following PYRapelin13 administration, consistent with a decrease (P = 0.048) in arrhythmia durations (13.1 ± 4.1 s vs. 3.3 ± 1.7 s). (J) Representative dominant frequency (DF) maps (Hz) generated from the time dependence of each pixel intensity subjected to a moving fast Fourier transform analysis. During AF, the local DF of electrical activity was observed within the RAA. Following PYRapelin13 administration, the DF in the RAA was eliminated, consistent with an inability to induce atrial arrhythmias. (K) Decreased arrhythmia inducibility following PYRapelin13 administration was associated with action potential duration prolongation (~17%, P < 0.001) at 80% of repolarization (APD80). APD80 also increased (~13%, P < 0.001) in sedentary mice, in which atrial arrhythmias cannot be induced. Data are presented as mean ± SEM. Scale bar: 200 μm. Representative data are the result of experiments being repeated at least 6 times. *P < 0.05, **P < 0.01, ***P < 0.001, paired and unpaired 2-tailed Student’s t tests, χ2 test, Mann-Whitney U or Wilcoxon signed-rank test comparing before and after PYRapelin13 administration.