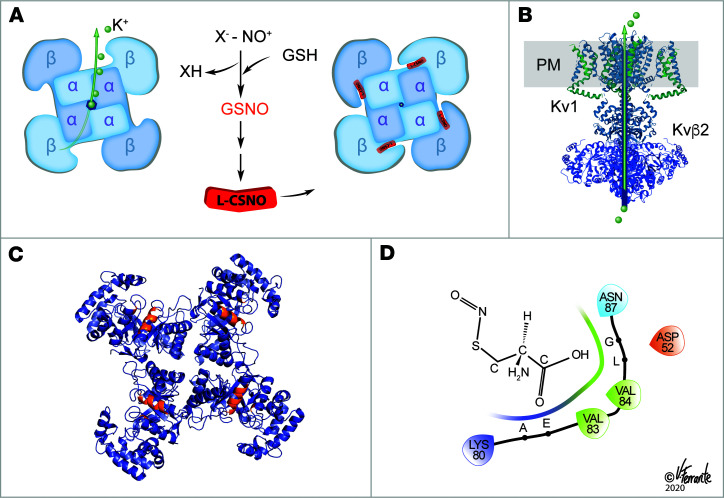
Figure 6. L-CSNO inhibition of voltage-gated K+ current.
(A) Top view: Schematic showing L-CSNO’s effect of altering the structure of the Kv multimer. The HDX data (Figure 3 and Supplemental Figure 3) show that the tertiary structure of Kvβ2 is altered by L-CSNO binding to make the protein more concave, particularly at the base near the site of its interaction with Kvα proteins. Kv1.1 T1 is also affected (Figure 3 and Supplemental Figure 3), but patch clamp data suggest that the interaction with Kvβ2 is essential for a functional effect (Figure 2). L-CSNO is formed from GSNO by γ-glutamyl transpeptidase and downstream dipeptidases (1), signaling increased VE (1). GSNO is formed, in turn, by NOS activation, oxyhemoglobin desaturation, ceruloplasmin and other metalloproteins capable of transferring NO+ equivalents to thiolate anions. Uniquely, NO is not transferred to the Kv proteins. D-CSNO does not share the activity of L-CSNO (Figures 2–5), though both isomers form NO at the same rate (20). (B) Lateral view of the Kvα/Kvβ complex in the plasma membrane, as in Figure 2A: side view of K+ exit from the cell. (C) Based on HDX, approximate sites of L-SNO binding in the 4 Kvβ2 subunits. (D) In silico estimates of L-CSNO interaction with Kvβ2 amino acids using ChemDraw and Schrödinger Maestro software. Reproduced with permission from V. Ferrante.