FIG 1.
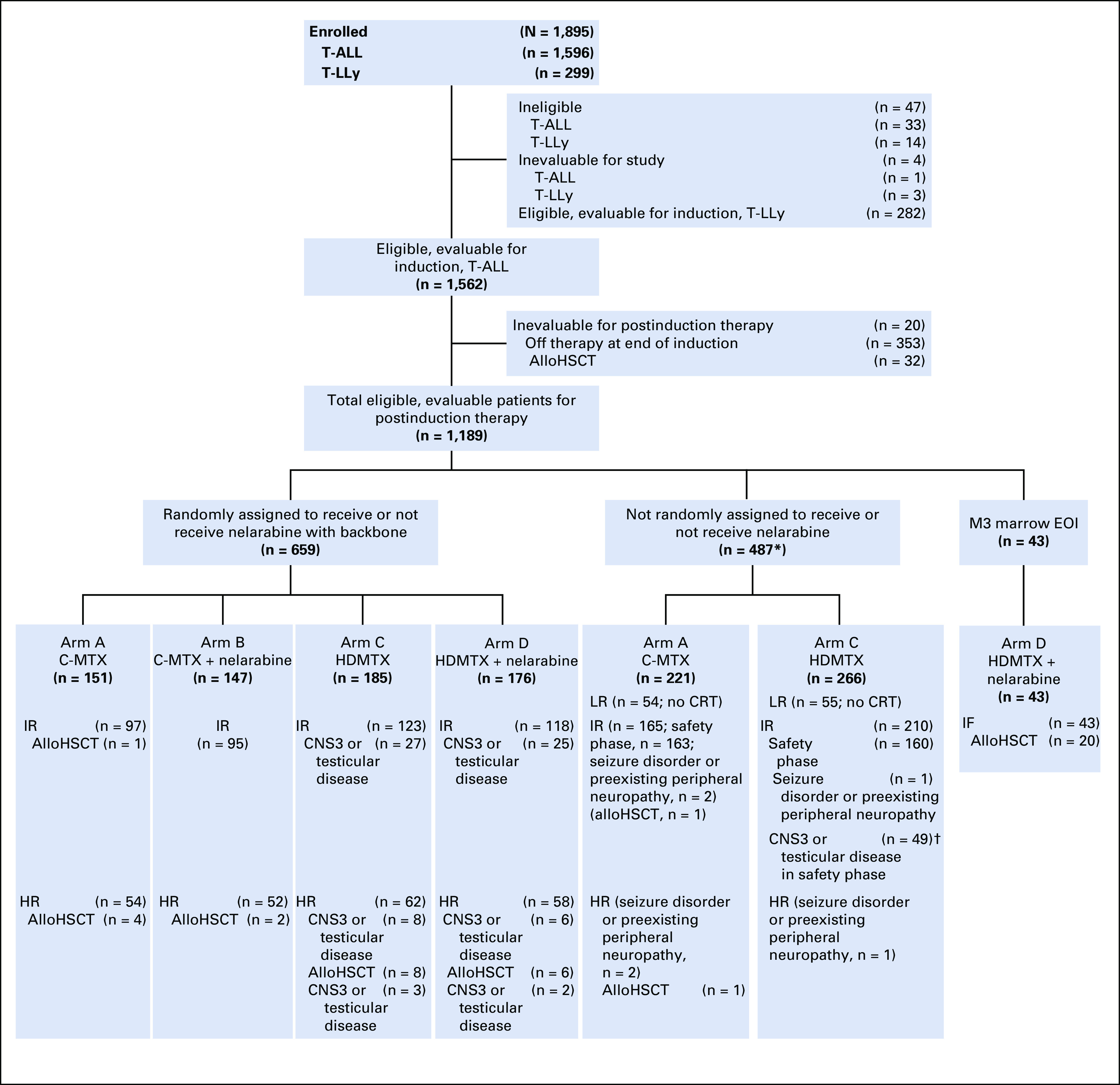
CONSORT diagram for study. (*) Includes patients who were not eligible to receive nelarabine (randomized to arms A and C only) either during the safety phase (intermediate risk [IR]) or efficacy phase (seizure disorder or preexisting peripheral neuropathy). (†) IR patients with CNS3 and testicular disease were assigned to arm C during the safety phase. The most commons reasons why patients came off study between the first and second stages of randomization were because the physician determined it was in the best interests of the patient and the participant declined to participate in the randomization. AlloHSCT, allogeneic hematopoietic stem-cell transplantation; C-MTX, escalating-dose methotrexate without leucovorin rescue plus pegaspargase; CRT, cranial radiation therapy; EOI, end of induction; HDMTX, high-dose methotrexate with leucovorin rescue; HR, high risk; IF, induction failure; LR, low risk; T-ALL, T-cell acute lymphoblastic leukemia; T-LLy, T-Cell lymphoblastic lymphoma.
