Figure 7.
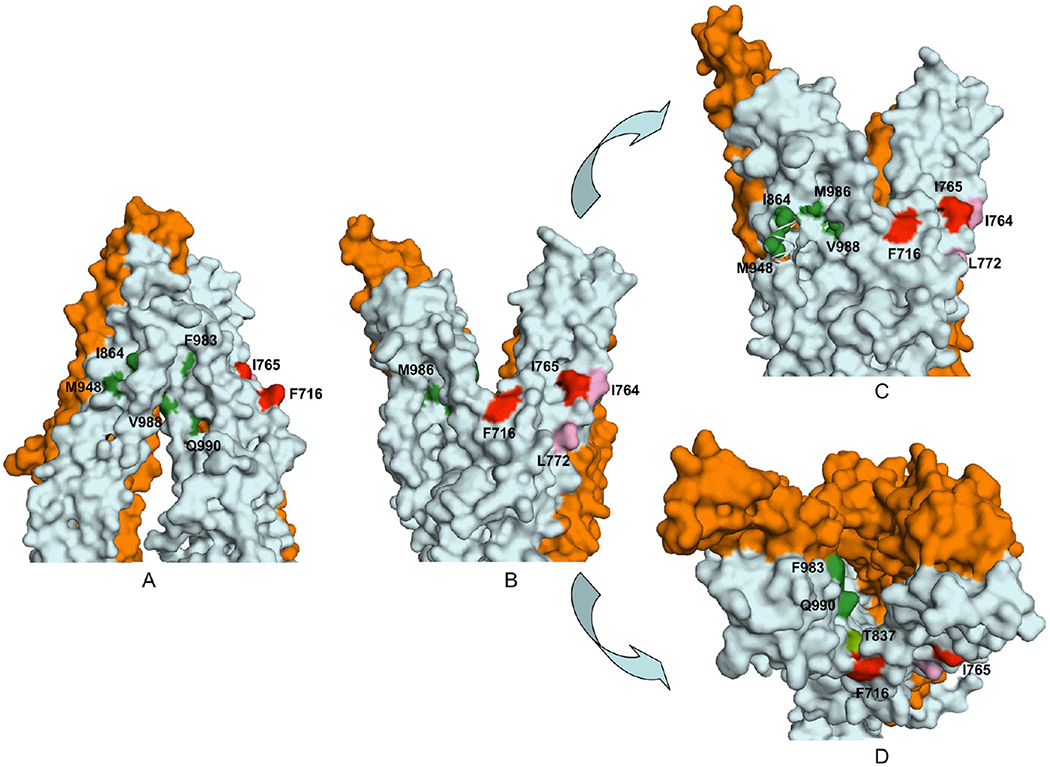
Surface contour diagram depicting the portal and the lipid-facing side pocket in relation to the modulator-specific residues. (A) Close-up view from parallel to the plane of the lipid bilayer of the TM regions, in the nucleotide-free state of Pgp, depicting the portal formed by a split between TM12 and TM10 and −11. (B) Same as panel A, except in the ADP-bound conformation showing formation of a side pocket with modulator-specific residues located inside it. (C) ADP-bound conformation with an ~15° clockwise rotation around the axis perpendicular to the plane of the lipid bilayer. The model shows part of TM10 removed to better visualize the modulator-specific residues located inside the side pocket. (D) View of panel B from the extracellular side of the membrane perpendicular to the plane of the lipid bilayer. The amino acid residues critical for ris-(Z)-flupentixol interaction (T837, I864, M948, F983, V988, M986, and Q990) are colored different shades of green and for verapamil red (F716 and I765) or pink (I764 and L772).
