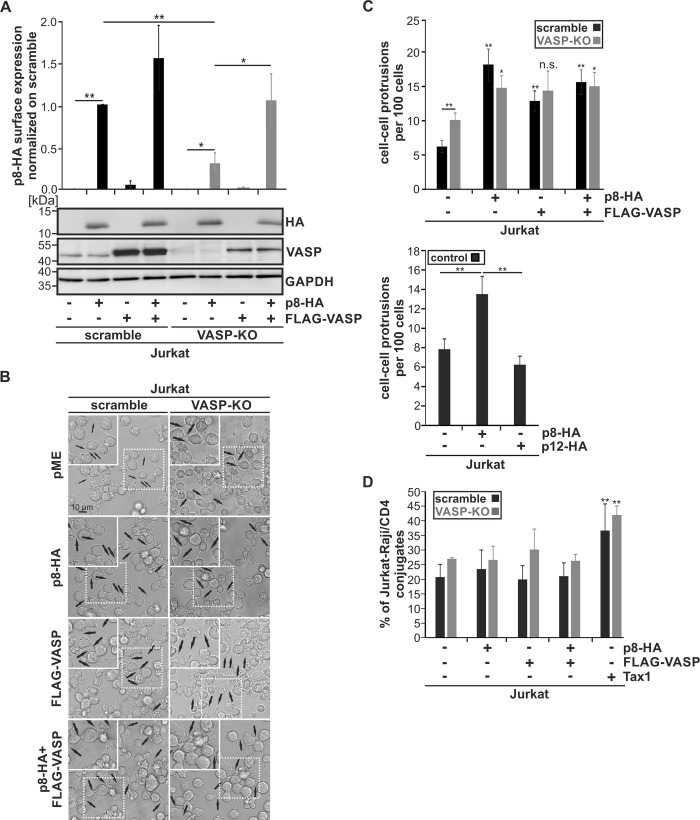
Fig 8. VASP is crucial for recruitment of p8 to the cell surface.
(A-D) Jurkat scramble or Jurkat VASP-KO cells were transfected with p8-HA, FLAG-VASP or both plasmids. Cells transfected with empty vectors pME and pEF-1α served as control. All samples were replenished with the respective empty vectors to 100 μg. At 48 h post transfection, (A) p8 surface expression, (B-C) cell-cell-protrusion formation, or (D) cell-cell conjugate formation were analyzed in at least three independent experiments. In parallel, protein lysates were isolated and subjected to Western blot analysis. (A) Transfected Jurkat scramble (black bars) and VASP-KO cells (grey bars) were stained without permeabilization using HA-specific, APC-labeled antibodies or the respective isotype-matched control antibodies and flow cytometry was performed. The mean fold change p8-HA surface expression of four independent experiments was compared using Student’s test (*, p<0.05; **, p<0.01). Additionally, a representative Western blot depicting p8-HA, VASP or the housekeeping gene GAPDH is shown. (B) Transfected cells were cultured without fixation in micro-slides for 1 h at 37°C and analyzed with a Leica TCS SP5 confocal laser scanning microscope equipped with a 63x1.4 HCX PL APO CS oil immersion objective lens. Transmitted light is shown. Black arrows indicate cell-cell-protrusions. Insets (dashed lines) are shown as enlargement (solid lines) (C) Quantitative analysis of data exemplified in (B) in Jurkat scramble (upper panel, black bars) and VASP-KO cells upper panel, dark grey bars), and as a control, in Jurkat cells (lower panel,black bars). In the latter case, cells transfected with p12-HA expression plasmids served as control. At least 20 optical fields per experimental condition were analyzed and mean numbers of cell-cell-protrusions per 100 cells were compared using Student’s t-test (*, p<0.05; **, p<0.01; n.s., not significant). (D) Conjugate formation between transfected Jurkat T-cells (scramble: black bars; VASP-KO, grey bars) and co-cultured Raji/CD4+ B-cells was quantitated by flow cytometry. Jurkat cells transfected with the Tax-expression construct pEF1α-Tax served as positive control. After 24h, Jurkat T-cells were co-cultured with Raji/CD4+ B-cells (ratio 1:1) for 1 h at 37°C. Co-cultures were fixed and stained with anti-CD3-AlexaFluor700 (for Jurkat T-cells) and anti-HLA-DR-PacificBlue antibodies (for Raji/CD4+ B-cells) to differentiate between the two cell types. Cell-cell conjugates were identified as double-positive signals (HLA-DR+CD3+) and normalized on the total number of Jurkat T-cells. The means of three independent experiments ± standard deviation are shown and were compared using Student's t-test (**, p<0.01).