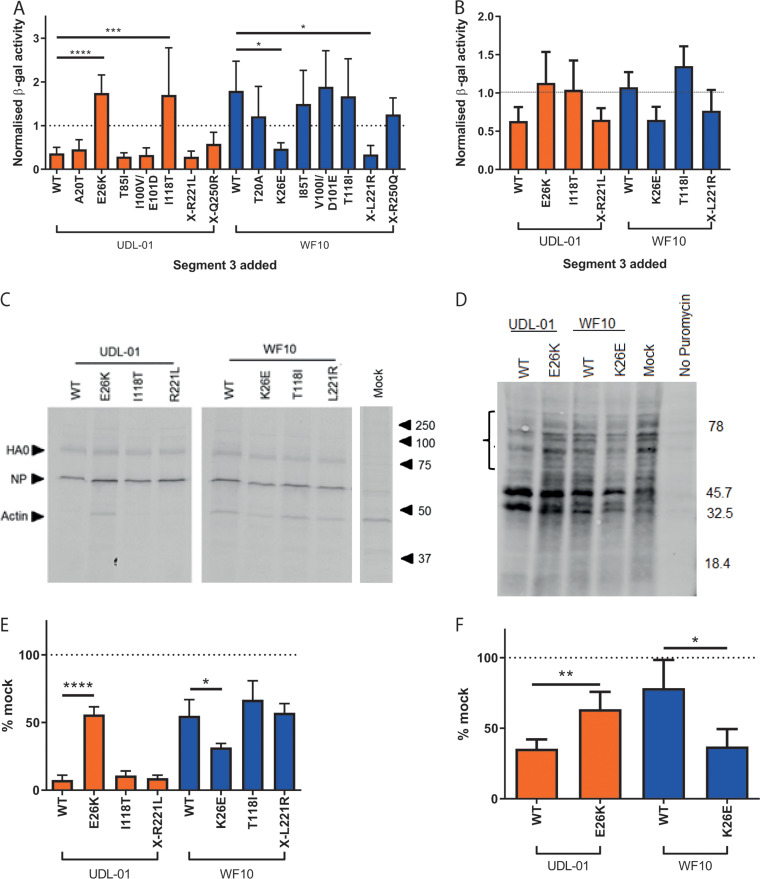
FIG 5.
Lysine at position 26 correlates with a lack of host shutoff activity in PA-X. WT or mutant segment 3 plasmids were cotransfected into (A) 293T cells or (B) DF-1 cells with a β-gal reporter plasmid. At 48 h posttransfection, cells were lysed and levels of β-gal assessed by colorimetric enzyme assay. Results were normalized to a sample where the PA plasmid was replaced with the empty vector control. Graph represents the average of 3 independent experiments ± SD. ****, P < 0.0001; ***, P < 0.0003; *, P < 0.003. (A, B) One-way analysis of variance (ANOVA) with multiple comparisons (all 293T and WF10 panel in DF-1) or Kruskal-Wallis with multiple comparisons (UDL-01 panel in DF-1). (C, D) Primary chicken embryonic fibroblasts (CEF) were infected with a high MOI (3) of virus. At 7 h postinfection, cells were pulsed with 35S methionine for 1 h, then lysed, and proteins were separated by SDS-PAGE. Radiolabeled proteins were detected by autoradiography. (C) Representative SDS-PAGE gel with specific proteins and the positions of molecular mass (kDa) markers indicated. (D) Levels of radiolabeled actin were quantified by densitometry using ImageJ analysis software. Graph represents the average of 3 independent experiments ±SD. *, P = 0.02; ****, P < 0.0001 (one-way ANOVA with multiple comparisons). (E, F) MDCK cells were infected with a high MOI (10) of each virus. 7.5 h postinfection cells were pulsed with puromycin for 30 min. Cells were lysed, run on SDS-PAGE gels, and Western blotted for puromycin. (E) Representative Western blot gel probed for puromycin. (F) The bracket in panel E covering the areas above 45.7 kDa indicates the region quantified using ImageJ analysis software to measure the area under the curve following densitometry of this region. Data were converted to a percentage of the value seen in mock-infected cells. Graph represents average ±SD of 3 independent experiments. *, P = 0.0124; **, P = 0.007 (unpaired t test).