Fig. 4. Structural characterization of open cryptic site mutant F143W.
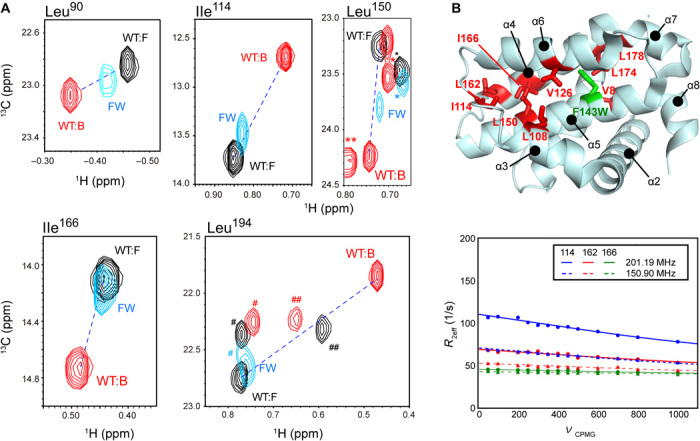
(A) Overlaid spectra of the unligated F143W mutant (cyan) and the unligated (black) and ABT-737–bound (red) WT Bcl-xL. Residues with substantial allosteric CSPs upon ABT-737 binding are shown. The peak positions of the residues that were not subjected to the analysis are indicated by *, **, #, and ## for Leu17, Leu90, Val10, and Leu162, respectively. Unligated and ABT-737–bound peaks are connected by a dashed line. (B) Methyl RD experiments with the unligated F143W mutant. The residues with substantial Rex (>5 Hz) contributions to the 13C methyl R2 were mapped on the structure of Bcl-xL, along with the mutation site. For the residues in the core of the cryptic site (Ile114, Leu162, and Leu166), the RD plots and their global fittings are shown.
