Fig. 3. Fluidic heating induces gene expression in 3D artificial tissues.
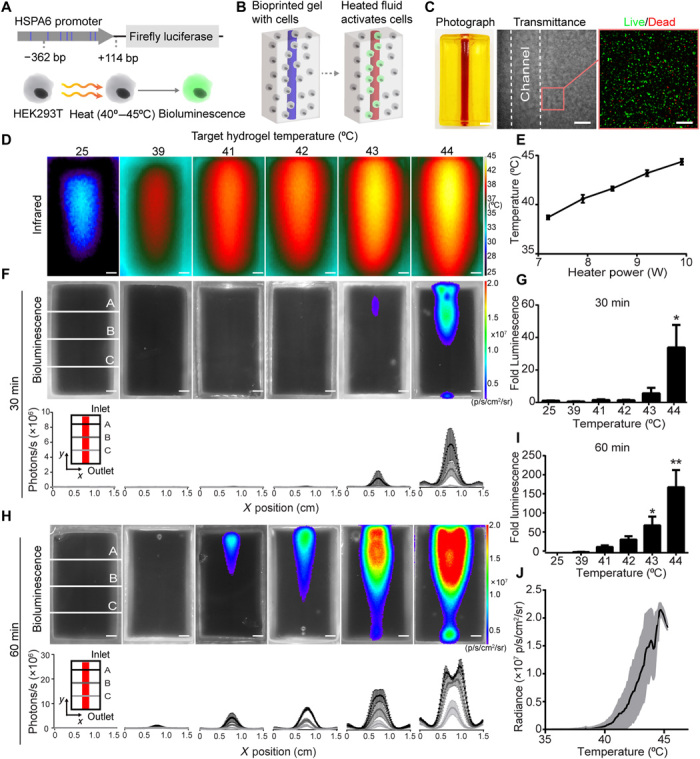
(A) HEK293T cells were engineered to express fLuc under the HSPA6 promoter. (B) Schematic of thermofluidic activation of encapsulated cells. (C) Single-channel tissue used for 3D heat activation (left). Scale bar, 3 mm. Transmittance image of cellularized hydrogel after printing (middle). Scale bar, 500 μm. HEK293T cells in bioprinted tissues stained with calcein-AM (“live,” green) and ethidium homodimer (“dead,” red; right). Scale bars, 200 μm. (D) Representative infrared images of thermofluidic perfusion in single-channel hydrogels. Scale bars, 2 mm. (E) Hydrogel temperatures are tuned by changing heater power at constant flow rate (n = 3, mean temperature ± standard error). (F) Representative bioluminescence images of hydrogels (top; scale bars, 2 mm) and intensity traces at three positions (A to C) across the width (x) of the hydrogel after 30 min of perfused heating. (G) Fold change in bioluminescence after 30 min of heating relative to 25°C controls. (H) Representative bioluminescence images of hydrogels (top; scale bars, 2 mm) and intensity traces after 60 min of perfused heating (bottom; scale bars, 2 mm). (I) Fold change in bioluminescence after 60 min of heating demonstrates a temperature-dependent dosage response in gene expression [(G and I); n = 3, mean fold luminescence ± standard error; *P < 0.05 and **P < 0.01 by one-way ANOVA followed by Dunnett’s multiple comparison test]. (J) Temperature-expression response curve (black) shows mean bioluminescent radiance across temperature; shaded regions (gray) indicate ± SD. n = 3. Photo credit: Daniel Corbett, University of Washington.
