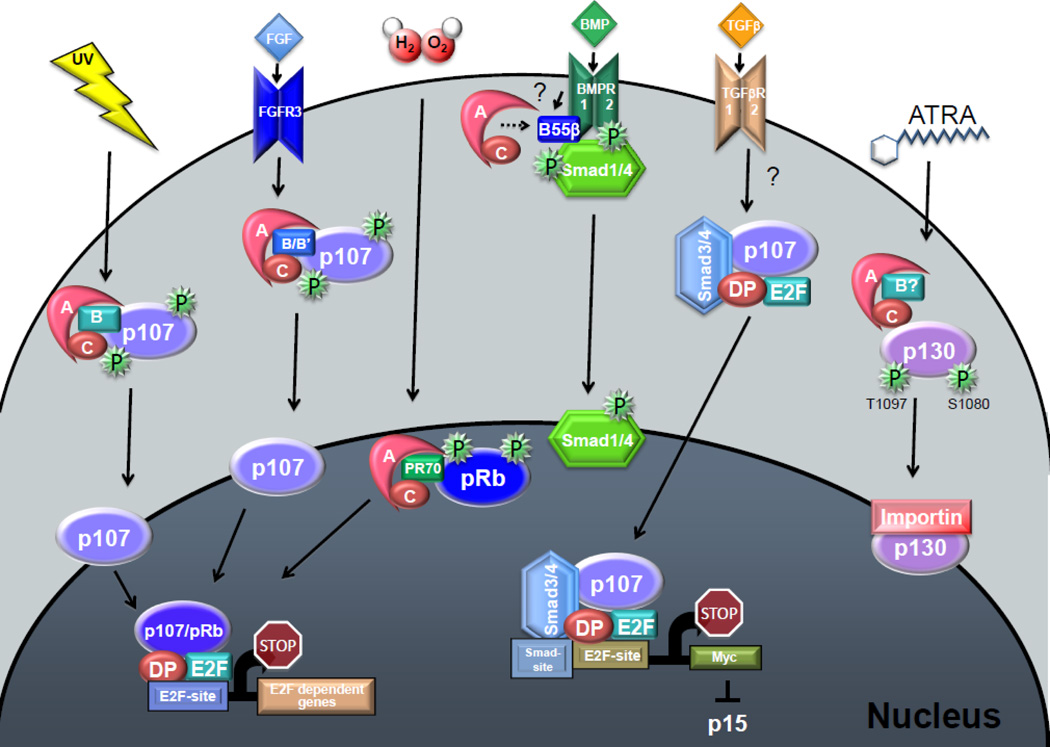
Figure 2. Upregulation of PP2A activity in response to various signals is associated with pocket protein dephosphorylation and cell cycle exit and maturation.
PP2A holoenzymes are activated in response to various signals including UV irradiation, FGF stimulation, H2O2 treatment, and BMP and all-trans-retinoic acid (ATRA) ligand/receptor binding. UV irradiation leads to specific dephosphorylation of p107, but the B subunit of the holoenzyme is unknown. FGF stimulation in chondrocytes leads to specific p107 dephosphorylation and the B subunit of the PP2A holoenzyme is likely a member of the B or B’ families. Oxidative stress results in pRB dephosphorylation by PR70 PP2A holoenzymes. While no specific phosphatase has been implicated in the formation of the p107/Smad/E2F complexes in response to TGFβ signaling (question mark), it was previously demonstrated that B55β interacts with the BMP receptor recruiting the PP2A/A–C dimer to ensure dephosphorylation of a linker site on SMADS that triggers nuclear translocation. Similar mechanisms could conceivably lead to TGFβ-mediated formation of the SMAD/p107/E2F/DP complex that translocates to the nucleus to repress Myc expression, in turn leading to derepression of p15 and growth arrest in epithelial cells. Finally, ATRA stimulates dephosphorylation of p130 and recruitment of p130 to de nucleus by importins. However, whether a B subunit is involved it is not known. Activation of pocket proteins via dephosphorylation results in complex formation with E2F/DP transcription factors leading to the repression of the E2F dependent gene program and eventual cell cycle exit. In these signaling pathways, association of pocket proteins with differentiation or maturation factors is also possible (see text for additional details).