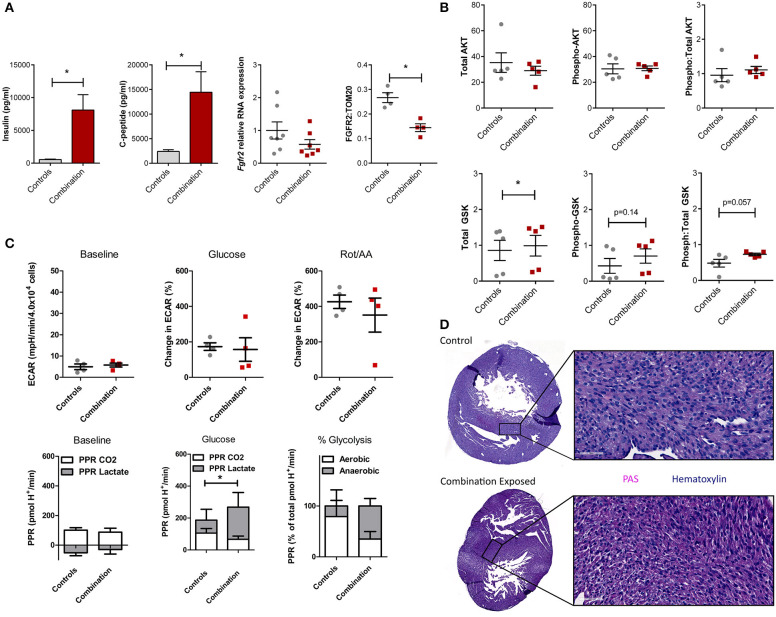
Figure 6.
Evidence of insulin resistance in diabetes and high fat diet exposed offspring hearts. Chronic exposure to insulin and other growth hormones can cause insulin resistance through downregulation of growth factor receptors and impaired downstream activation of the PI3K/AKT pathway which shifts metabolism from glycolysis to gluconeogenesis/glycogen accumulation. (A) Combination exposed newborn offspring (n = 10/group) had significantly higher circulating insulin and c-peptide levels. Consistent with transcriptome analyses, combination exposed newborn male hearts, had a trend toward lower RNA expression relative to B2m (p = 0.13, n = 7/group) and lower protein expression (p < 0.05, n = 4/group) of FGFR2. (B) Total and phosphorylated AKT (n = 5/group) was not different, but GSK3β was higher (n = 5/group) and there was a trend toward more phosphorylated (active) and ratio of phosphorylated:total GSK3β (p = 0.14 and p = 0.057, respectively) in combination exposed, male hearts. (C) Primary isolated newborn rat cardiomyocytes (NRCM) from combination exposed male offspring had no significant difference in baseline extracellular acidification rate (ECAR), glucose or rotenone/antimycin (Rot/AA) stimulated glycolysis (glycolytic capacity) by XF analyses (top row). The proton production rate (PPR) was calculated to estimate aerobic (PPR from CO2) and anaerobic (PPR from lactate) glycolysis. At baseline, there was no difference, but aerobic glycolysis was significantly lower in combination exposed NRCM following glucose injection. Combination exposed NRCM had only 34% aerobic glycolysis vs. 79% aerobic glycolysis following glucose in controls. This suggests maternal diabetes and high fat diet exposure impairs aerobic glycolytic capacity. *p < 0.05, n = NRCM pooled from 3 to 4 pups/litter, 4 litters/group. (D) Periodic Acid Schiff (PAS) staining demonstrates more glycogen deposition in combination exposed hearts, which suggests a chronic in utero switch from glucose utilization to storage occurred during development.