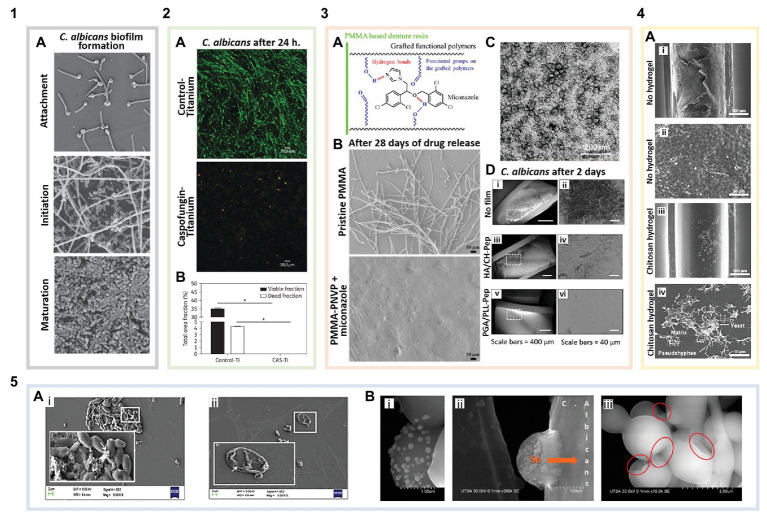
Figure 2.
Biomaterials for the prevention and treatment of Candida biofilms. (1) Biofilm formation of non-functionalized surfaces: (1A) Candida albicans biofilm formation (adapted with permission from Ramage et al., 2009). (2) Surface-tethered antifungals: (2A) Live/Dead staining showing caspofungin functionalized titanium disks (with caspofungin surface coverage of ~2,191 pmol/cm2) inhibiting C. albicans attachment and biofilm formation compared to bare titanium (green are live cells, and red indicates membrane compromised cells; adapted with permission from Kucharíková et al., 2016). (2B) Quantification of viable cells per area in the images shown in 2A (adapted with permission from Kucharíková et al., 2016). (3) Antifungal loaded polymer coatings: (3A) Schematic illustrating miconazole-polymer hydrogen bonding (adapted with permission from Wen et al., 2016a). (3B) Miconazole loaded into poly(methyl methacrylate)-poly(1-vinyl-2-pyrrolidinone) (PMMA-PNVP) films inhibits C. albicans attachment and biofilm growth for up to 28 days compared to pristine PMMA (adapted with permission from Wen et al., 2016a). (3C) Antifungal poly(ethylene glycol) (PEG) + curcumin (CU) nanocomposites in solution after being released from graphene oxide (GO) coatings (adapted with permission from Devadas et al., 2019). (3D) Layer-by-layer (LbL) coated catheters prevent C. albicans attachment and biofilm formation after 2 days. (i) Uncoated catheters showing C. albicans attachment and biomass deposition. (ii) Magnified region outlined in 3D(i). (iii) Catheters coated with a hyaluronic acid (HA)/chitosan (CH) LbL film with β-peptide showing no C. albicans attachment but some biomass deposition on the surface. (iv) Magnified region outlined in 3D(iii). (v) Poly-L-glutamic acid (PGA)/poly-L-lysine (PLL) LbL film with β-peptide coated catheters showing no cell or biomass attachment. (vi) Magnified region outlined in 3D(v) (adapted with permission from Raman et al., 2016). (4) Inherently antifungal polymer coatings: (4A) Polyurethane catheter-associated Candida parapsilosis biofilms. (i) Uncoated catheters exhibiting Candida attachment and biofilm formation. (ii) Magnified region of image 4A(i) showing a dense C. parapsilosis biofilm. (iii) Catheters coated with low molecular weight CH hydrogels significantly reduce Candida cell attachment and biofilm formation. (iv) Magnified region of image 4A(iii) showing biofilm disruption (adapted with permission from Silva-Dias et al., 2014). (5) Antifungal nanoparticles: (5A) Scanning electron microscopy (SEM) images of C. albicans biofilms on polystyrene. (i) Control biofilm cells [white arrow points to extracellular polymeric substances (EPSs)] and (ii) biofilm inhibition in the presence of ferulic acid-chitosan nanoparticles (white arrow indicates the damaged fungal cell wall; adapted with permission from Panwar et al., 2016). (5B) SEM images of selenium nanoparticles (i,ii) binding to and (iii) disrupting C. albicans cells in biofilms. The red circles indicate areas of the cell membrane, where the nanoparticles have induced shrinking and folding (adapted with permission from Guisbiers et al., 2017).