Abstract
High-fat diets (HFDs) adversely influence glutamate metabolism and neurotransmission. The precise role of the group II metabotropic glutamate receptors (mGluR2/3) antagonist on spatial memory deficit following consumption of HFD has not yet been clarified. Therefore, in this study, we examined the effects of post-training administration of mGluR2/3 antagonism; LY341495 on spatial memory in rats fed with HFD (for 10 weeks) by using Morris Water Maze (MWM) task. The training session for testing memory acquisition in MWM consisted of 4 trials per day for 4 consecutive days. Twenty-four hours after the last training session the spatial probe test (retention) was given. Intraperitoneal injection (i.p) injection of LY341495 was done 30 min before probe test. Our results showed that 10 weeks consumption of HFD had no significant effect on escape latency and swimming distance in memory acquisition. Our finding showed that consumption of a HFD leads to reference memory impairment in the probe test. HFD animals spent less time in the target zone in compare with control animals. Also, LY341495 improved HFD-induced reference memory (retention) impairment. HFD animals treated with LY341495 spent more time in the target zone in compare with HFD animals. Escape latencies to find the visible platform during visual task were same in all experimental groups, indicating no visual impairment in the animals. We propose that a HFD may act through mGluR2/3 within the brain to reduce synaptic plasticity, which impairs memory retrieval, and post-training administration of LY341495 can reduce HFD-induced reference memory impairment.
Keywords: Metabotropic Glutamate Receptors (mGluR2/3), LY341495, Spatial memory, High-fat diets, Rat
1. Introduction
Despite the role of nutrients and foods in public health, information about the real effects of high-fat diet (HFD) on central nervous system (CNS) structure and function is still poor. Consuming an HFD has long been known to raise the risk of some diseases such as obesity, diabetes mellitus, metabolic syndrome, Alzheimer’s disease and some types of cognitive impairment in humans and rodents (Eskelinen et al., 2008). Although many studies repeatedly have shown that HFD is associated with learning and memory impairment, it has recently been reported that HFD improves memory in Wistar rats (Setkowicz et al., 2015). These observations show a contradictory effect of HFD on learning and memory. And it seems that further studies are necessary to clarify the issue. Thus, prospective studies evaluating the effects of HFD on cognitive functions are needed.
It is possible that HFD and obesity may induce cognitive disruption by impacting glutamate metabolism and neurotransmission. The glutamate actions are mediated by ionotropic and metabotropic glutamate receptors (iGluRs and mGluRs respectively) (Niswender and Conn, 2010; Karimi et al., 2015; Traynelis et al., 2010). The mGluRs are G-protein coupled receptors and have eight subtype and are classified into three Groups (I (mGluR1 and mGluR5), II (mGluR2 and mGluR3), and III mGluR4, mGluR6, mGluR7, and mGluR8) depending on their signal transduction pathways, sequence homology, and pharmacological selectivity. It has been shown that HFDs alter glutamate metabolism and neurotransmission in mice hippocampus and downregulates the NMDA receptor subunit NR2B (Valladolid-Acebes et al., 2012). Also, in our previous work we examined the induction of long-term potentiation (LTP) under the blockade of the mGluR2/3 in the hippocampal perforant path- dentate gyrus (DG) pathway in rats fed an HFD (Karimi et al., 2015). Interestingly we observed that blocking of group II mGluRs increased LTP in rats fed an HFD.
Modulation of glutamate transmission using compounds acting at mGluRs offers a hopeful way to investigate the role of the glutamatergic system in cognitive signs following consumption of HFD, and may promote the development of novel treatments for these cognitive impairments. mGluRs have a vital role in mediating glutamatergic system in the brain (Niswender and Conn, 2010). Group II receptors are located mainly in presynaptic terminals (Cartmell and Schoepp, 2000) and are highly expressed in CNS areas related to learning and memory, such as the hippocampus, amygdala, and prefrontal cortex (PFC) (Ohishi et al., 1993a, b). Moreover, cognitive enhancement has been observed after treatment with metabotropic glutamate receptor 2/3 antagonists and glutamatergic blockade in some studies (Gargiulo et al., 2005; Hondo et al., 1994). The present study, therefore further explored the effects of metabotropic glutamate receptor 2/3 manipulation on cognitive performance.
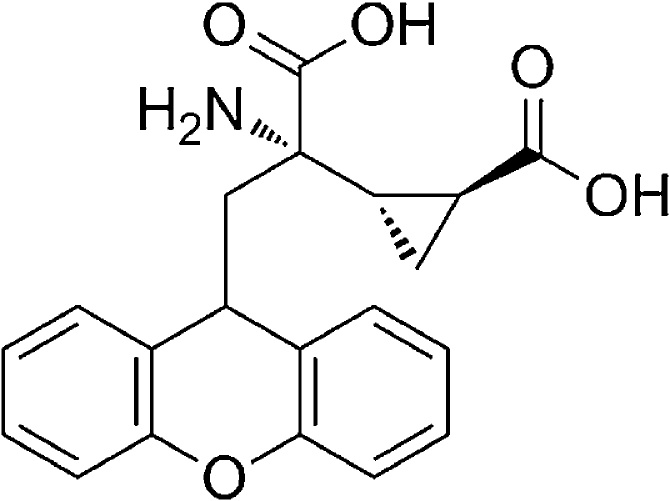
The precise role of mGlu2/3 receptor antagonists on spatial memory deficit following consumption of HFD has not yet been clarified. Therefore, in this study, we examined the effects of post-training administration of mGluR2 and 3 antagonism; LY341495 (Fig. 1) on spatial memory in rats fed with HFD by using Morris Water Maze (MWM) task.
Fig. 1.
LY341495 Chemical structure. Potent and selective group II metabotropic glutamate receptors (mGluR2/3) antagonist.
2. Materials and methods
2.1. Ethics statement
All experimental procedures using rats were conducted in accordance with the animal care and use guidelines approved by the institutional ethics committee at Hamadan University of Medical Sciences and were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2. Animals, LY341495 administration and experimental design
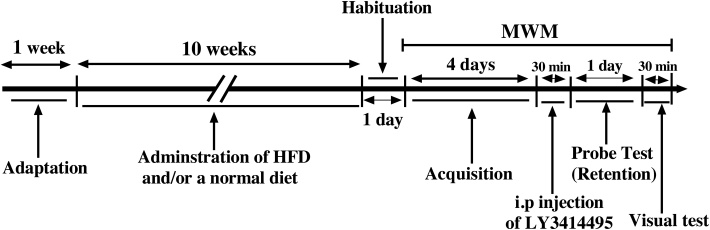
Thirty-two adult male Wistar rats weighing 250−300 g obtained from Pasteur Institute of Tehran, Iran. The animals were housed in an air-conditioned room at 22 ± 2 °C with a 12-h light/dark cycle. The animals were kept in cages with 2–3 rats in each cage. Standard animal chow and water were freely available. After one week of adaptation, subjects were randomly divided into following groups (N = 6–8): control group consumed an ordinary diet; HFD group received high-fat diet only; control + LY group: control animals treated with LY341495; HFD + LY group: HFD animals treated with LY341495. Animals were maintained on standard diet or HFD regimes for 10 weeks before subjecting them for MWM test. After 10 weeks, MWM task was used to evaluate the spatial learning and memory in rats. We dissolved the selective and highly potent antagonist for mGluR2/3, (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495, Tocris) in ∼10 μL of 0.1 M NaOH and brought to dose volume using 0.9 % sterile saline solution. A single intraperitoneal injection (i.p) of LY341495 (3 mg/kg) as an antagonist of mGluR2/3 was done 30 min before retention test (Bespalov et al., 2007; Linden et al., 2005; Pitsikas et al., 2012; Barker et al., 2006). The LY341495 dose was selected on the basis of results from previous published studies that evaluated the effects of this compound on cognition in mice and rat (Bespalov et al., 2007; Linden et al., 2005; Pitsikas et al., 2012; Barker et al., 2006; Gregory et al., 2003). Experimental design and schedule of MWM task are shown in Fig. 2.
Fig. 2.
Experimental design and schedule of behavioral tests. After one week of adaptation, animals were maintained on standard diet or HFD regimes as per the protocol for 10 weeks before subjecting them for testing using MWM. After 10 weeks, MWM task was used to evaluate the spatial learning and memory in rats. i.p injection of LY3414495 as an antagonist of mGluR2/3 was done 30 min before retention test.
2.3. Diet composition
Rats were fed with standard rat food pellets for 7 days in order to recover from transportation. The HFD was designed according to the standard HFD D12492 Dietary, which is used for develop obesity (Furnes et al., 2009). Firstly, Pellet dry powder was mixed slowly for 15 min using an electric mixer. Subsequently, animal fatty oils (8.3 %), hydrogenated oil (4.05 %), cholesterol (1%), and sugar (17/3%) were added to the mixture of powder and were mixed for 15 min. Pellet dry powder contains various compounds such as minerals, proteins and amino acids as follows: calcium (0.95 %), Phosphorus (0.65 %), magnesium (0.47 %), zinc (0.05 %), iodine (0.15 %), Lysine (1. 5 %), methionine (0.33 %), cysteine (0.63 %), threonine (0.72 %), tryptophan (0.25 %), crude protein (23 %), crude fat (4 %), crude fiber (4 %), and crude ash (10 %). An HFD has a caloric density of approximately 5.24 kcal/g. A standard laboratory rodent chow diet (Lab Diet) was used for the control diet. This control diet has a caloric density of approximately 3.0 kcal/g.
2.4. Morris water maze (MWM) task
2.4.1. Apparatus
MWM task is a hippocampal-dependent test of spatial learning for rodents (Karimi et al., 2017; Hajisoltani et al., 2019). The MWM task is a relatively simple procedure. The main advantage being the differentiation between the spatial (hidden-platform) and non-spatial (visible platform) conditions. In addition, the MWM testing environment reduces odor trail interference. This has led the task to be used extensively in the study of the neurobiology and neuropharmacology of spatial learning and memory. The MWM plays an important role in the validation of rodent models for neurocognitive disorders such as Alzheimer’s disease (Bromley-Brits et al., 2011). Because of importance and advantage of MWM task, we used MWM for evaluation of learning and memory deficit in rats fed with high-fat diet. The water maze apparatus consisted of a black-painted circular pool, (155 cm diameter, 60 cm height), filled to a depth of 35 cm with water (22 ± 1 °C). The pool was divided into four equal quadrants. A hidden platform (10 cm in diameter), made of Plexiglas, was located 2 cm under the water surface in the center of the eastern quadrant (target quadrant). A video-computer tracking system (CCD camera, Panasonic Inc., Japan) was used to record the rats' swim path for later analysis (EthoVision software XT7, Netherland). Large posters on the wall of the room served as visual cues.
2.4.2. Habituation
Twenty-four hours before starting the hidden platform training, rats were given a 60 s swim in the tank without platform for adaptation to the environment.
2.4.3. Hidden platform training
The training session was done according to the previous procedure conducted in our laboratory (Karimi et al., 2017; Omidi et al., 2019; Karimi et al., 2019; Rezagholizadeh et al., 2020). In summary, training session was consisted of one block of 4 trails per day for four consecutive days. Each trial was started by placing the animal in one of the four quadrants. Animals were allowed to swim the in pool during a period of 90 s to find the hidden platform that was kept in the middle of one of the four quadrants. If an animal did not find the platform within this period, it was manually guided to the platform by the investigator. The rats rested 10 min between the two consecutive trials. The escape latency (i.e., time to reach the platform) and swimming distance were used to assess acquisition of the water maze task. Swimming speed was used to assess the motor activity of the rats. In our work, we used daily average of all trials from day 1 to day 4, and analyzed them.
2.4.4. Spatial reference memory (probe test or retention)
Twenty-four hours after the 4th session the spatial probe test was given. In the spatial probe test, the platform was removed, and rats were allowed to swim for 60 s before they were removed. Animals were released in the water in a location that was exactly opposite from where the platform was placed in the hidden platform training sessions. Behavior was recorded with a video tracking system. Time spent in target zone was recorded for subsequent analysis.
2.4.5. Visual test
Thirty minutes after the probe trial, the platform was elevated above the water surface; covered by bright color aluminum foil, and placed in a different zone and rats were allowed to swim and find the visible platform during 60 s in order to test their visual ability. All experiments were conducted between 10:00 and 12:00.
2.5. Statistical analysis
Data were presented as mean ± SEM and processed by commercially available software GraphPad Prism® 8.0.2. In the behavioral study (MWM), the data of the training trials were analyzed using a two-way analysis of variance (ANOVA) with days as repeated measures factor and treatments as between subjects’ factor. For statistical analyses of probe and visibility trial data, one-way ANOVA was used. Two-way and one-way ANOVA were followed by post hoc analysis (Bonferoni and Tukey’s tests, respectively). The level P < 0.05 was considered as statistically significant.
3. Results
3.1. Consumption of HFD for 10 weeks had no effect on memory acquisition
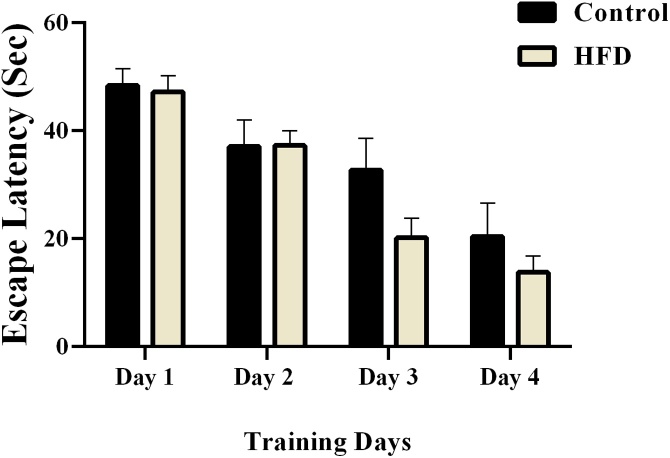
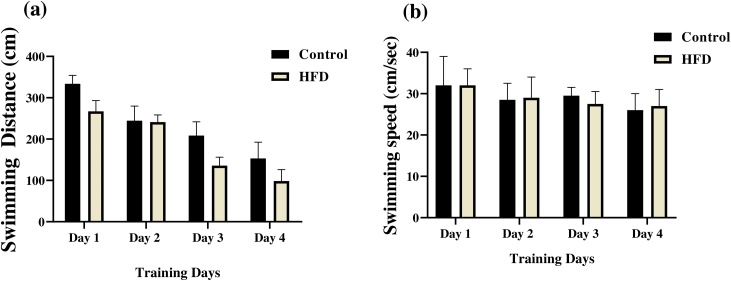
All animals learned the location of the hidden platform after 4 days of training. Escape latency decreased significantly (control rats: F (2.786, 19.50) = 12.53, P = 0.0001, HFD group: F (2.216, 15.51) = 30.60, P < 0.0001, repeated measures one-way ANOVA) after 4 days when compared to the first day (Fig. 3). Swimming distance also decreased (Fig. 4a) but swimming speed did not reveal remarkable change during training (p > 0.05, (Fig. 4b).
Fig. 3.
Effects of high-fat diet on escape latency in the training days of water maze task. A decrease in the escape latency was observed over time in both the control group and HFD group. We used daily average of all trials from day 1 to day 4. Data presented as means ± S.E.M.
Fig. 4.
Effects of high-fat diet on swimming distance (a) and swimming speed (b) in the training days of water maze task. A decrease in the travel distance was observed over time in both the control group and HFD group, but swimming speed did not reveal remarkable change during training. We used daily average of all trials from day 1 to day 4. Data presented as means ± S.E.M.
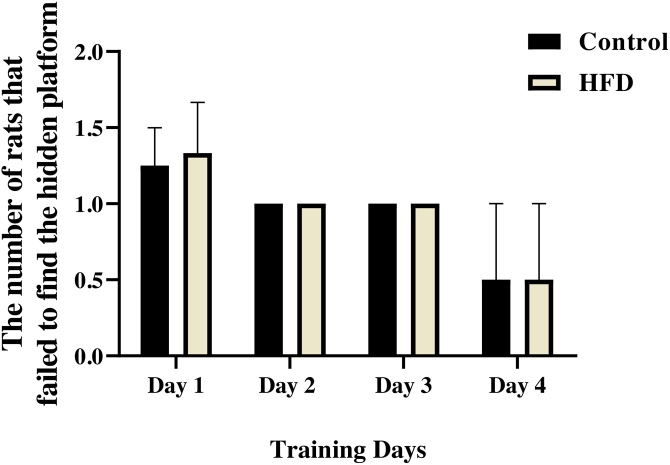
There were not significant differences in Escape latency between experimental groups (Treatment effect: F (1, 14) = 1.184, P = 0.2948, Day effect; F (2.693, 37.70) = 36.75, P < 0.0001, Interaction; F (3, 42) = 1.754, P = 0.1706, two-way ANOVA, Fig. 3). In addition, there no statistically significant differences in swimming distance between experimental groups (Treatment effect: F (1, 16) = 2.416, P = 0.1397, Day effect; F (2.938, 47.00) = 33.42, P < 0.0001, Interaction; F (3, 48) = 1.405, P = 0.2528, Fig. 4a). Also, the number of rats that could not find the hidden platform (within each training day) for 90 s is shown in Fig. 5, which was not statistically significant.
Fig. 5.
The number of rats that could not find the hidden platform (within each training day) for 90 s. There was no statistically significant difference between the groups during the training days. Data presented as means ± S.E.M.
3.2. LY341495 reduce HFD-induced reference memory impairment
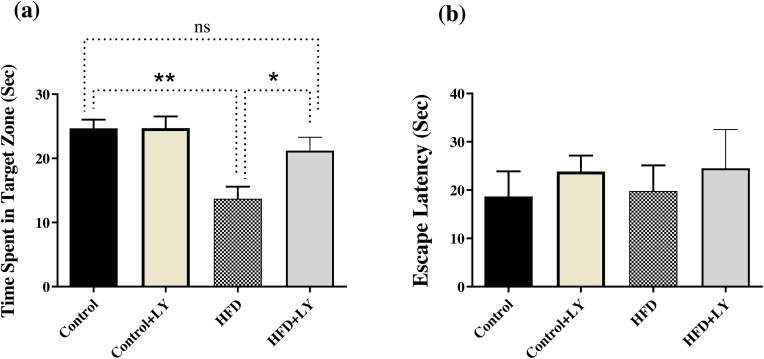
To assess reference memory at the end of learning, a probe trial was conducted 24 h after the last training trial on day 5. During this test, the platform was removed and time spent in each quadrant of the MWM was recorded. Our results showed that consumption of an HFD leads to spatial memory impairment. There were significant differences in time spent in target zone between experimental groups [F (3, 20) = 7.031, P = 0.0021, n = 6–8, one-way ANOVA, Fig. 6a]. HFD animals spent less time in the target zone in compare with control animals (control rats: 24. ± 1.36 s, n = 6 control + LY rats: 24.6 ± 2.5 s, n = 6, HFD rats: 13.7 ± 1.28 s n = 8). Also, LY341495 improved HFD-induced reference memory impairment. HFD animals treated with LY341495 spent more time in the target zone in compare with HFD animals (P = 0.0449).
Fig. 6.
Spatial reference memory was measured in a probe test. Animals fed with a HFD spent less time in the target zone (a). HFD animals treated with LY341495 spent more time in the target zone in compare with HFD animals. Animals in all groups showed no difference in escape latency (b) to find a visible platform. Data presented as means ± S.E.M. * p < 0.05, ** p < 0.01, ns; not significant.
LY341495 and HFD had no effect on swimming speed in the probe test indicating no motor disturbances occurred in the animals (data not shown, p > 0.05, one-way ANOVA). In the visible test taken after probe test, all animals could find the platform (Fig. 6b). Escape latencies to find the visible platform during visual discrimination task were the same in all experimental groups (F (3, 21) = 0.2114, P = 0.8874, one-way ANOVA, Fig. 6b), indicating no visual impairment in the animals.
4. Discussion
Using an MWM task in rat, the present study showed that 10 weeks feeding with HFD had no effect on memory acquisition but impaired retrieval of reference memory and induced amnesia. Moreover, HFD induced impairment of memory was restored by pre-test administration of LY341495.
Unlike most previous studies; our results established that spatial learning ability in HFD-treated rats remained intact. Some studies have even shown that consumption an HFD improves memory.
In this regard, Zuzanna and colleagues observed that long-term HFD for 12 months improved the learning ability (Setkowicz et al., 2015). They also found that prolonged HFD consumption leads to higher hippocampal volume and higher hippocampal metabolite concentrations, possibly due to increased ketone bodies levels in the blood. Moreover, it has been reported that that increase in the humans body mass index is associated with improvement in spatial abilities (Gunstad et al., 2010). Also, it has been demonstrated that HFD alters gut microbiota but not spatial working memory in early middle-aged Sprague Dawley rats (Deshpande et al., 2019). Haleem and Mahmood have reported that HFD improves learning acquisition and memory retention but impair reference memory (Haleem and Mahmood, 2019).
Consistent with previous findings, our results verified that HFD induced impairment of memory retrieval which was restored by post training administration of LY341495. Cognitive improvement has been observed after treatment with mGluR2/3 antagonists and glutamatergic blockade in some studies (Gargiulo et al., 2005; Hondo et al., 1994). mGluR2/3 act as inhibitory autoreceptors in the presynaptic glutamatergic terminals, which inhibit the release of glutamate (Cavoy and Delacour, 1993). Therefore, activation of mGluR2/3 reduces glutamate signaling, whereas mGluR2/3 antagonism increases it. High expression of mGlu2/3 receptors have been observed in the hippocampus and amygdala (Ohishi et al., 1993a, b). It is known that these brain areas are involved in learning and memory processing, although mixed results have been obtained (Ennaceur and Delacour, 1988). So, all these regions may be potential sites of LY341495 action.
mGluR2/3 antagonist increase release of glutamate and levels of serotonin in the PFC (Kawashima et al., 2005). It is proposed that increased levels of serotonin may lead to enhanced memory consolidation (Harmer et al., 2002).
Consistent with our results previous studies have reported that mGluR2/3 antagonists improves spatial memory task and acquisition of a visual discrimination (Gargiulo et al., 2005; Higgins et al., 2004).
As noted in the introduction section, in our previous work we found that blocking of group II mGluRs increases LTP in rats fed an HFD (Karimi et al., 2015). These results indicated that mGluR2/3 inhibition may have stimulatory effects on LTP induction in the hippocampal perforant path- DG pathway. As a result, at least one of the basic mechanisms for memory improvement effects of LY341495 following HFD consumption appears to be its enhancing effect on LTP. Blockade of mGluR2/3 reduces the harmful effects of an HFD on LTP and possibly improves memory. LTP is a major form of synaptic plasticity in the CNS, thought to underlie learning and memory (Bliss and Collingridge, 1993) and has been reported that LTP is impaired by HFD consumption (Karimi et al., 2013; Salehi et al., 2018). Also, it has been shown that mGluRs are involved in various forms of synaptic plasticity in the hippocampus (Bortolotto et al., 1999). Underwood and Thompson have found that HFD significantly reduces hippocampal intrinsic excitability in both young adult male and female Long-Evans rats (Underwood and Thompson, 2016), and activation of group II mGluRs increases the hippocampal network activity (Ster et al., 2011).
It has been shown that LY341495 administration prevents Aβ-induced neurodegeneration (Caraci et al., 2011). Also, LY341495 administration into PFC showed a non-significant trend toward increased T-maze test performance in rats (Gregory et al., 2003) and even facilitated performance in working and spatial memory tasks in rats. In addition, post-training administration of LY341495 coupled with administration of 2-methyl 6- (phenylethyl) -pyridine (MPEP) as a mGluR5 antagonist improved MPEP's amnestic effect on recognition memory (Barker et al., 2006). In contrast, it has been reported that LY341495 administration causes impairment in passive avoidance task and habituation in mice (Bespalov et al., 2007; Sato et al., 2004) and the LY341495 differentially affected recognition memory in rats (Pitsikas et al., 2012).
The above studies show the different effects of LY341495 on various cognitive functions. To our knowledge, no studies have addressed the effects of LY341495 on the post-training components of spatial memory in rats fed an HFD. Collectively, our results propose a role for mGluR2/3 antagonists in the handling of memory impairment following consumption of HFD.
Acute administration of LY341495 has been reported to stimulate locomotor activity in mice (O’Neill et al., 2003). But in our work, swimming speed was not different among the experimental groups, demonstrating that LY341495 did not affect locomotor activity. The contradictory observation between our work and others may be due to differences in type of the animal used (subjects), variations in study design, experiment conditions, and LY341495 dosage used. In addition, it has been shown that LY341495 did not influence the locomotor activity in rats (Pitsikas et al., 2012).
The current work proposes that 10 weeks consumption of an HFD can impair spatial memory and mGluR2/3 inhibition maybe have restored the harmful effects of HFD on memory. We propose that an HFD may act through mGluR2/3 within the brain to reduce synaptic efficacy and modify synaptic plasticity (Karimi et al., 2015). Our data suggest that HFD might have a significant effect on cognitive function by promoting neurochemical changes affecting glutamate metabolism.
5. Conclusion
In conclusion, our results suggested that 10 weeks consumption of HFD has no effects on the acquisition of spatial learning, but can impair memory retention of the adult male rats and post-training administration of LY341495 can improve HFD-induced reference memory impairment. The different processes are involved in the acquisition and retrieval of memory. Retrieval tests result not only in reactivating the memory from acquisition, but also in establishing new memories for the events that occur during the retrieval tests themselves (Abel and Lattal, 2001). Also, distinct molecular mechanisms are involved in memory acquisition and retrieval (Abel and Lattal, 2001). And the effect of consuming HFD on acquisition and retrieval of memory may be different.
Conflicts of interest
The authors report no declarations of interest.
Ethics statement
All experimental procedures using rats were conducted in accordance with the animal care and use guidelines approved by the institutional ethics committee at Hamadan University of Medical Sciences and were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Authors’ contributions
SAK designed the project, wrote the manuscript and performed the statistical analysis, revised the manuscript and supervised the project. HM, EH, IS and HS were involved in laboratory works and experimental design of the work. AS, AK and JK were involved in data collection and lab assessments, and study designing. All authors read and approved the final manuscript for publication.
CRediT authorship contribution statement
Heresh Moridi: Investigation, Data curation, Writing - review & editing. Abdolrahman Sarihi: Resources, Funding acquisition, Data curation, Writing - review & editing, Validation. Elahe Habibitabar: Investigation, Formal analysis, Data curation, Writing - review & editing. Hossein Shateri: Investigation, Data curation, Writing - review & editing. Iraj Salehi: Resources, Funding acquisition, Data curation, Writing - review & editing, Validation. Alireza Komaki: Resources, Funding acquisition, Data curation, Project administration, Writing - review & editing, Validation. Jamshid Karimi: Resources, Funding acquisition. Seyed Asaad Karimi: Supervision, Conceptualization, Formal analysis, Data curation, Funding acquisition, Writing - original draft, Writing - review & editing, Validation.
Acknowledgments
The study was funded by Vice-chancellor for Research and Technology and Student Research Center, Hamadan University of Medical Sciences (No. 9805013348, Code of Ethics Committee: IR.UMSHA.REC.1398.323). The authors would like to express their gratitude to the staff of the Neurophysiology Research Center for helping them carry out this project.
References
- Abel Ted, Lattal K. Matthew. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Barker, Isaac Gareth Robert, Bashir Zafar Iqbal, Brown Malcolm Watson, Warburton Elizabeth Clea. A temporally distinct role for group I and group II metabotropic glutamate receptors in object recognition memory. Learn. Mem. 2006;13:178–186. doi: 10.1101/lm.77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov Anton, Jongen-Rêlo Ana-Lucia, van Gaalen Marcel, Harich Silke, Schoemaker Hans, Gross Gerhard. Habituation deficits induced by metabotropic glutamate receptors 2/3 receptor blockade in mice: reversal by antipsychotic drugs. J. Pharmacol. Exp. Ther. 2007;320:944–950. doi: 10.1124/jpet.106.110684. [DOI] [PubMed] [Google Scholar]
- Bliss Tim V.P., Collingridge Graham L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bortolotto Zuner A., Fitzjohn Stephen M., Collingridge Graham L. Roles of metabotropic glutamate receptors in LTP and LTD in. Curr. Opin. Neurobiol. 1999;9:299–304. doi: 10.1016/s0959-4388(99)80044-0. [DOI] [PubMed] [Google Scholar]
- Bromley-Brits Kelley, Deng Yu, Song Weihong. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Visual. Exp. 2011;53 doi: 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraci Filippo, Molinaro Gemma, Battaglia Giuseppe, Giuffrida Maria Laura, Riozzi Barbara, Traficante Anna, Bruno Valeria, Cannella Milena, Merlo Sara, Wang Xushan. Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer’s disease: selective activation of mGlu2 receptors amplifies β-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol. Pharmacol. 2011;79:618–626. doi: 10.1124/mol.110.067488. [DOI] [PubMed] [Google Scholar]
- Cartmell J., Schoepp D.D. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Cavoy A., Delacour J. Spatial but not object recognition is impaired by aging in rats. Physiol. Behav. 1993;55:527–530. doi: 10.1016/0031-9384(93)90148-9. [DOI] [PubMed] [Google Scholar]
- Deshpande Nikita Girish, Saxena Juhi, Pesaresi Tristan G., Dylan Casey, Carrell, Ashby Grayson Breneman, Liao Min-Ken, Freeman Linnea Ruth. High fat diet alters gut microbiota but not spatial working memory in early middle-aged Sprague Dawley rats. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A., Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Eskelinen Marjo H., Ngandu Tiia, Helkala Eeva‐Liisa, Tuomilehto Jaakko, Nissinen Aulikki, Soininen Hilkka, Kivipelto Miia. Fat intake at midlife and cognitive impairment later in life: a population‐based CAIDE study. Int. J. Geriatric Psychiatry. 2008;23:741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- Furnes M.W., Zhao C.M., Chen D. Development of obesity is associated with increased calories per meal rather than per day. A study of high-fat diet-induced obesity in young rats. Obes. Surg. 2009;19:1430–1438. doi: 10.1007/s11695-009-9863-1. [DOI] [PubMed] [Google Scholar]
- Gargiulo Pascual A., Acerbo Martin Javier, Krug Ines, Delius J.D. Cognitive effects of dopaminergic and glutamatergic blockade in nucleus accumbens in pigeons. Pharmacol. Biochem. Behav. 2005;81:732–739. doi: 10.1016/j.pbb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Gregory Mary L., Stech Nicholas E., Owens Russell W., Kalivas Peter W. Prefrontal group II metabotropic glutamate receptor activation decreases performance on a working memory task. Ann. N. Y. Acad. Sci. 2003;1003:405–409. doi: 10.1196/annals.1300.037. [DOI] [PubMed] [Google Scholar]
- Gunstad John, Lhotsky April, Wendell Carrington Rice, Ferrucci Luigi, Zonderman Alan B. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34:222–229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajisoltani Razieh, Karimi Seyed Asaad, Rahdar Mona, Davoudi Shima, Borjkhani Mehdi, Hosseinmardi Narges, Behzadi Gila, Janahmadi Mahyar. Hyperexcitability of hippocampal CA1 pyramidal neurons in male offspring of a rat model of autism spectrum disorder (ASD) induced by prenatal exposure to valproic acid: a possible involvement of Ih channel current. Brain Res. 2019;1708:188–199. doi: 10.1016/j.brainres.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Haleem Darakhshan Jabeen, Mahmood Khalid. Brain serotonin in high-fat diet-induced weight gain, anxiety and spatial memory in rats. Nutr. Neurosci. 2019:1–10. doi: 10.1080/1028415X.2019.1619983. [DOI] [PubMed] [Google Scholar]
- Harmer Catherine J., Bhagwagar Zubin, Cowen Phillip J., Goodwin Guy M. Acute administration of citalopram facilitates memory consolidation in healthy volunteers. Psychopharmacology. 2002;163:106–110. doi: 10.1007/s00213-002-1151-x. [DOI] [PubMed] [Google Scholar]
- Higgins Guy A., Ballard Theresa M., Kew James N.C., Grayson Richards J., Kemp John A., Adam Geo, Woltering Thomas, Nakanishi Shigetada, Mutel Vincent. Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology. 2004;46:907–917. doi: 10.1016/j.neuropharm.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Hondo Hisao, Yonezawa Yuji, Nakahara Tatsuo, Nakamura Kaoru, Hirano Makoto, Uchimura Hideyuki, Tashiro Nobutada. Effect of phenycyclidine on dopamine release in the rat prefrontal cortex; an in vivo microdialysis study. Brain Res. 1994;633:337–342. doi: 10.1016/0006-8993(94)91558-x. [DOI] [PubMed] [Google Scholar]
- Karimi Seyed Asaad, Salehi Iraj, Komaki Alireza, Sarihi Abdolrahman, Zarei Mohammad, Shahidi Siamak. Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: an in vivo study. Brain Res. 2013;1539:1–6. doi: 10.1016/j.brainres.2013.09.029. [DOI] [PubMed] [Google Scholar]
- Karimi Seyed Assad, Komaki Alireza, Salehi Iraj, Sarihi Abdolrahman, Shahidi Siamak. Role of group II metabotropic glutamate receptors (mGluR2/3) blockade on long-term potentiation in the dentate gyrus region of hippocampus in rats fed with high-fat diet. Neurochem. Res. 2015;40:811–817. doi: 10.1007/s11064-015-1531-3. [DOI] [PubMed] [Google Scholar]
- Karimi Seyed Asaad, Hosseinmardi Narges, Janahmadi Mahyar, Sayyah Mohammad, Hajisoltani Razieh. The protective effect of hydrogen sulfide (H2S) on traumatic brain injury (TBI) induced memory deficits in rats. Brain Res. Bull. 2017;134:177–182. doi: 10.1016/j.brainresbull.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Karimi Seyed Asaad, Salehi Iraj, Shykhi Teimor, Zare Samad, Komaki Alireza. Effects of exposure to extremely low-frequency electromagnetic fields on spatial and passive avoidance learning and memory, anxiety-like behavior and oxidative stress in male rats. Behav. Brain Res. 2019;359:630–638. doi: 10.1016/j.bbr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Kawashima Naoya, Karasawa Jun-ichi, Shimazaki Toshiharu, Chaki Shigeyuki, Okuyama Shigeru, Yasuhara Akito, Nakazato Atsuro. Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neurosci. Lett. 2005;378:131–134. doi: 10.1016/j.neulet.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Linden A.-M., Bergeron M., Schoepp D.D. Comparison of c-Fos induction in the brain by the mGlu2/3 receptor antagonist LY341495 and agonist LY354740: evidence for widespread endogenous tone at brain mGlu2/3 receptors in vivo. Neuropharmacology. 2005;49:120–134. doi: 10.1016/j.neuropharm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Niswender Colleen M., Conn P.Jeffrey. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill Michael F., Heron-Maxwell Claire, Conway Michael W., Monn James A., Ornstein Paul. Group II metabotropic glutamate receptor antagonists LY341495 and LY366457 increase locomotor activity in mice. Neuropharmacology. 2003;45:565–574. doi: 10.1016/s0028-3908(03)00232-6. [DOI] [PubMed] [Google Scholar]
- Ohishi H., Shigemoto R., Nakanishi S., Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi Hitoshi, Shigemoto Ryuichi, Nakanishi Shigetada, Mizuno Noboru. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Omidi Ghazaleh, Karimi Seyed Asaad, Rezvani-Kamran Arezoo, Monsef Amirreza, Shahidi Siamak, Komaki Alireza. Effect of coenzyme Q10 supplementation on diabetes induced memory deficits in rats. Metab. Brain Dis. 2019:1–8. doi: 10.1007/s11011-019-00402-7. [DOI] [PubMed] [Google Scholar]
- Pitsikas N., Kaffe E., Markou A. The metabotropic glutamate 2/3 receptor antagonist LY341495 differentially affects recognition memory in rats. Behav. Brain Res. 2012;230:374–379. doi: 10.1016/j.bbr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezagholizadeh Amir, Karimi Seyed Asaad, Hosseinmardi Narges, Janahmadi Mahyar, Sayyah Mohammad. The effects of glial cells inhibition on spatial reference, reversal and working memory deficits in a rat model of traumatic brain injury (TBI) Int. J. Neurosci. 2020:1–14. doi: 10.1080/00207454.2020.1807544. [DOI] [PubMed] [Google Scholar]
- Salehi Iraj, Komaki Alireza, Karimi Seyed Asaad, Sarihi Abdolrahman, Zarei Mohammad. Effect of garlic powder on hippocampal long-term potentiation in rats fed high fat diet: an in vivo study. Metab. Brain Dis. 2018;33:725–731. doi: 10.1007/s11011-017-0174-2. [DOI] [PubMed] [Google Scholar]
- Sato Tomoaki, Tanaka Koh-ichi, Ohnishi Yoshiko, Teramoto Toyonori, Irifune Masahiro, Nishikawa Takashige. Inhibitory effects of group II mGluR-related drugs on memory performance in mice. Physiol. Behav. 2004;80:747–758. doi: 10.1016/j.physbeh.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Setkowicz Zuzanna, Gaździńska Agata, Osoba Joanna J., Karwowska Karolina, Majka Piotr, Orzeł Jarosław, Kossowski Bartosz, Bogorodzki Piotr, Janeczko Krzysztof, Wyleżoł Mariusz. Does long-term high fat diet always lead to smaller hippocampi volumes, metabolite concentrations, and worse learning and memory? A magnetic resonance and behavioral study in wistar rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ster Jeanne, María Mateos José, Grewe Benjamin Friedrich, Coiret Guyllaume, Corti Corrado, Corsi Mauro, Helmchen Fritjof, Gerber Urs. Enhancement of CA3 hippocampal network activity by activation of group II metabotropic glutamate receptors. Proc. Natl. Acad. Sci. 2011;108:9993–9997. doi: 10.1073/pnas.1100548108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis Stephen F., Wollmuth Lonnie P., McBain Chris J., Menniti Frank S., Vance Katie M., Ogden Kevin K., Hansen Kasper B., Yuan Hongjie, Myers Scott J., Dingledine Ray. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood Erica L., Thompson Lucien T. High-fat diet impairs spatial memory and hippocampal intrinsic excitability and sex-dependently alters circulating insulin and hippocampal insulin sensitivity. Biol. Sex Differ. 2016;7:9. doi: 10.1186/s13293-016-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladolid-Acebes I., Merino B., Principato A., Fole A., Barbas C., Lorenzo M.P., García A., Del Olmo N., Ruiz-Gayo M., Cano V. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Endocrinol. Metab. Am. J. Physiol. 2012;302:396–402. doi: 10.1152/ajpendo.00343.2011. [DOI] [PubMed] [Google Scholar]