FIG 7.
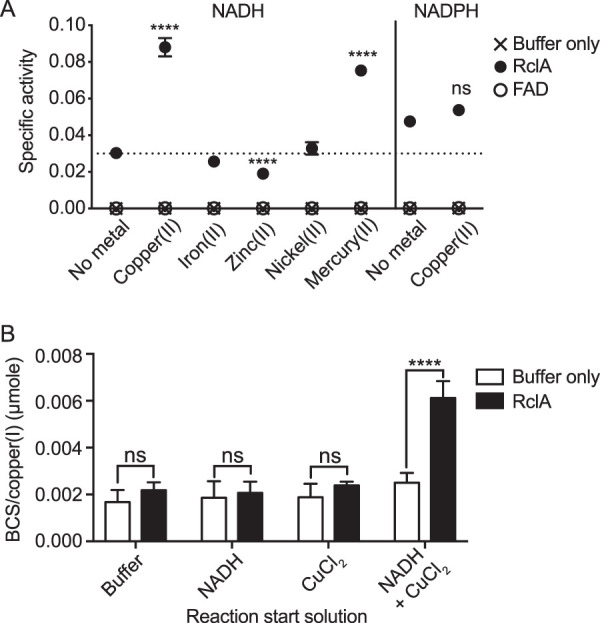
RclA reduces Cu(II) to Cu(I). (A) RclA specific activity (SA) increases in the presence of Cu(II) and Hg(II). SA [μmol of NAD(P)+ min−1 mg−1 RclA] of RclA was assayed by measuring NAD(P)H oxidation over time spectrophotometrically (n = 6 for each reaction type, ± the SD). Reactions were started by adding 100 μl of NAD(P)H or NADH and each indicated metal (both to 200 μM final) to 100 μl of RclA (3 μM final) at 37°C using the injector system of a Tecan Infinite M1000 plate reader. All reactions were carried out in 20 mM HEPES–100 mM NaCl (pH 7). NADH absorbance at 340 nm was measured each minute for 5 min. “Buffer only” denotes NAD(P)H oxidation in the presence of the indicated metals, and the “FAD” reactions were performed with 3 μM FAD to control for any possible free cofactor that may contribute to metal reduction in the RclA positive reactions. Differences in SA in the presence of each metal were analyzed using a two-way ANOVA with Dunnett’s multiple-comparison test using the no metal reaction as the control (****, P < 0.0001; **, P < 0.01). (B) Cu(I) accumulates after the RclA and NADH/copper (II) reaction, as measured by BCS/Cu(I)-complex absorption. Each reaction described in panel A was then stopped at 5 min with 10 μl of a BCS (400 μM final) and EDTA (1 mM final) solution using the injector system of the plate reader. The stopped reaction mixtures were incubated at 37°C for 5 min, with the absorbance of BCS/Cu(I) complex being measured at 483 nm each minute to ensure saturation of BCS. Differences in the amount of BCS/Cu(I) complex between the buffer-only and RclA reactions were analyzed using a two-way ANOVA with Dunnett’s multiple-comparison test using the buffer-only sample as the control for each reaction start solution (****, P < 0.0001; ns, not significant).
