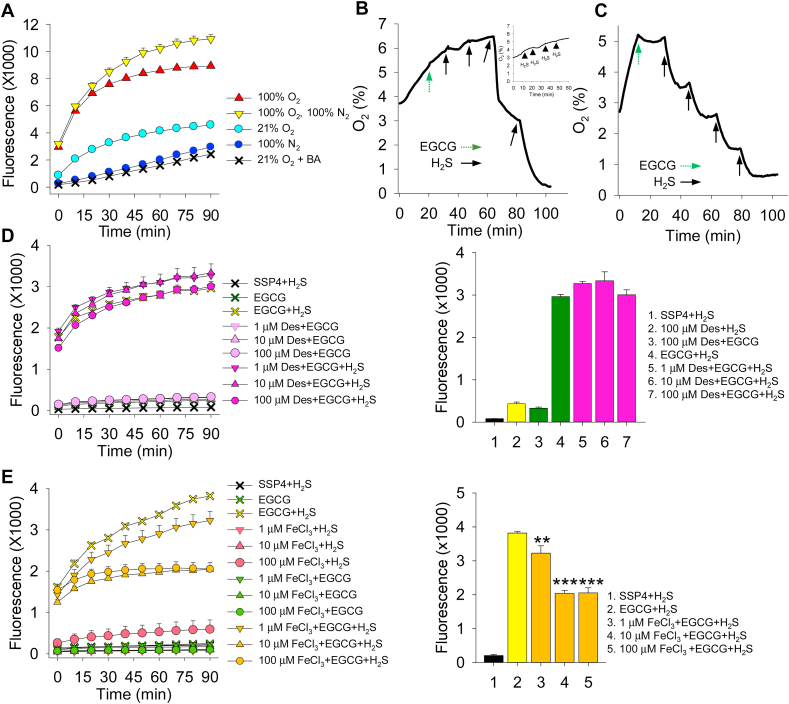
Fig. 7.
Role of oxygen, boric acid (BA) and iron in EGCG oxidation of H2S to polysulfides. (A) EGCG (100 μM) was incubated in buffer bubbled for 2 h with either 100% O2, room air (21% O2), 100% N2 (100% O2), or 100% O2 for 2 h then bubbled with 100% N2 for 10 min (100% O2, 100% N2) and subsequently exposed to 300 μM H2S for 1 h. Oxygen increased polysulfide production; little polysulfide production was observed in buffer bubbled with 100% N2 and incubated with H2S for 1 h or in 21% O2 buffer with BA (100 mM) for 1 h. (B) Typical trace showing O2 consumption after addition of 100 μM EGCG (dashed arrow) and 1 mM H2S (solid arrows) at pH 7.4. EGCG slightly decreased the rate of O2 increase, whereas H2S progressively decreased O2 concentration. Inset shows that H2S alone did not substantially affect O2 levels. (C) Typical trace showing O2 consumption after addition of 100 μM EGCG (dashed arrow) and 1 mM H2S (solid arrows) in buffer at pH 9.0. The effects of EGCG and H2S were greatly amplified compared to pH 7.4 (D) Desferrioxamine from 1 to 100 μM did not affect polysulfide formation by EGCG reaction with 100 μM H2S. (E) FeCl3 decreases polysulfide formation by EGCG and 100 μM H2S. Mean + SE, n = 4 wells per treatment; **, P < 0.01; ***, P < 0.001; error bars may be hidden by symbols. Bar graphs show effects at 90 min incubation with SSP4.