Figure 1. Crystal structure of eukaryotic Uba4 at 2.2 Å resolution.
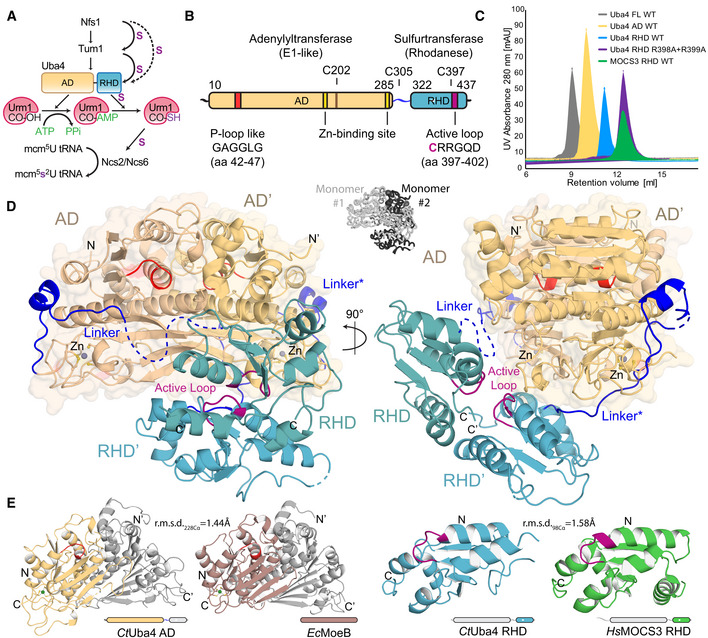
- Scheme of the cytoplasmic tRNA thiolation pathway. Nfs1 mobilizes the sulfur and transfers it via a persulfide onto the rhodanese‐like domains (RHDs) of either Tum1 or Uba4. Uba4 catalyzes the activation and thiocarboxylation of Urm1. Urm1‐COSH is used by the Ncs2/Ncs6 complex to form 2‐thiouridine on tRNAs. AD: adenylation domain. RHD: rhodanese‐like domain.
- Domain organization of CtUba4. AD: adenylation domain. RHD: rhodanese‐like domain.
- Size‐exclusion chromatography profiles of recombinant proteins used in this study. AU: arbitrary units; WT: wild type; FL: full‐length.
- Cartoon representation of the CtUba4 dimer. The AD dimer is emphasized by a transparent surface representation, and disordered loops are shown as dashed lines. The AD and RHD active loops are colored in red and purple, respectively. Zn atoms are shown as grey spheres. The two interwound Uba4 monomers are shown in black and white (central inset).
- Structural comparison of CtUba4 AD with EcMoeB (PDB ID: 1JW9) and of CtUba4 RHD with HsMOCS3 RHD (PDB ID: 3I2V). r.m.s.d. values as a measure of structural similarity are indicated.
