Figure 5. The unconventional Uba4–Urm1 reaction scheme and its comparison to canonical UBLs.
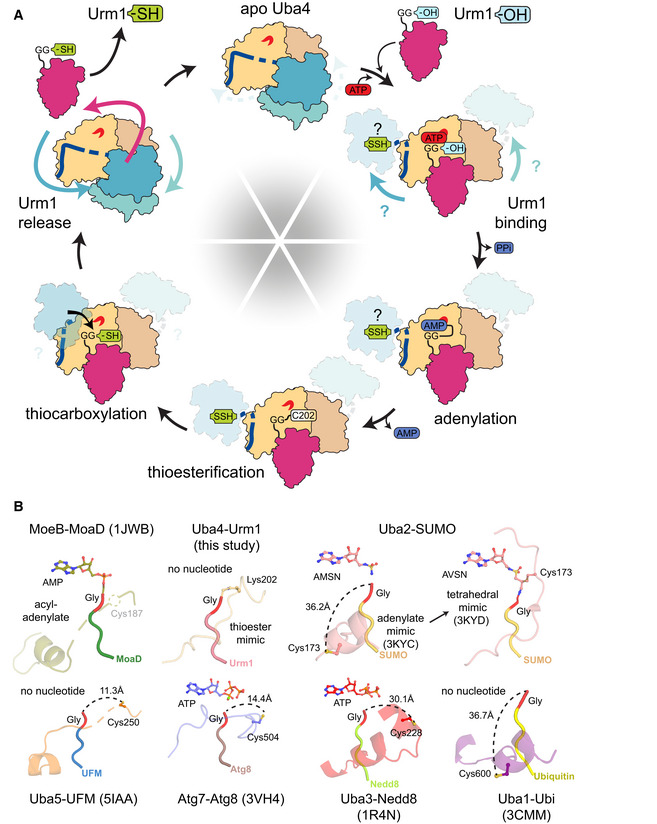
- Working model of the Uba4‐catalyzed reaction cycle of Urm1 thiocarboxylation. After adenylation by the Uba4 AD (ochre), Urm1 (red) forms a thioester intermediate with the catalytic cysteine (C202) located within the crossover loop. The RHD (blue) connected to the AD by a flexible linker reaches Urm1 and replaces the thioester linkage by an acyl‐disulfide bond by a nucleophilic attack of the persulfide on the active cysteine (C397). Thiocarboxylated Urm1 is released and serves as sulfur donor for tRNA thiolation.
- Structural comparison of various E1‐UBL system focusing on the position of their catalytic cysteine residues, crossover loops, and nucleotides. Distances between C‐terminal glycine (red) and catalytic cysteine residues are indicated unless they are covalently linked. PDB identifiers, nucleotide types, and residue numbers are labeled.
