Figure EV2. Known PI(4,5)P2‐binding sites in FAK support membrane placement.
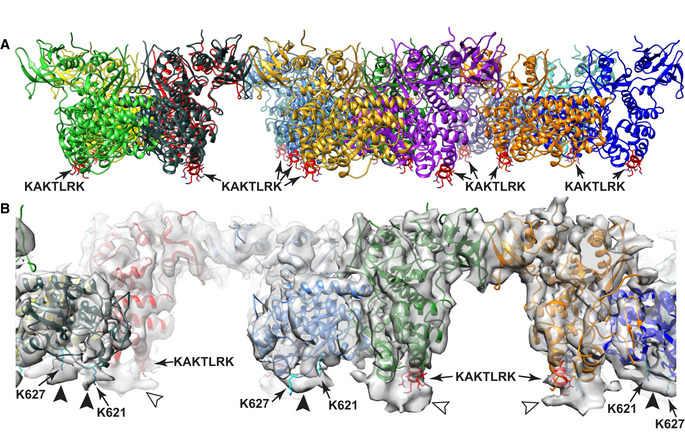
- Unmodeled density at the KAKTLRK sites (red) in FERM domains (light arrowheads) and unmodeled density at K621/K627 residues (cyan) in the kinase domains (dark arrowheads) coincide with the position of the modeled membrane and likely represent lipid molecules with restricted mobility due to their interactions with FAK.
