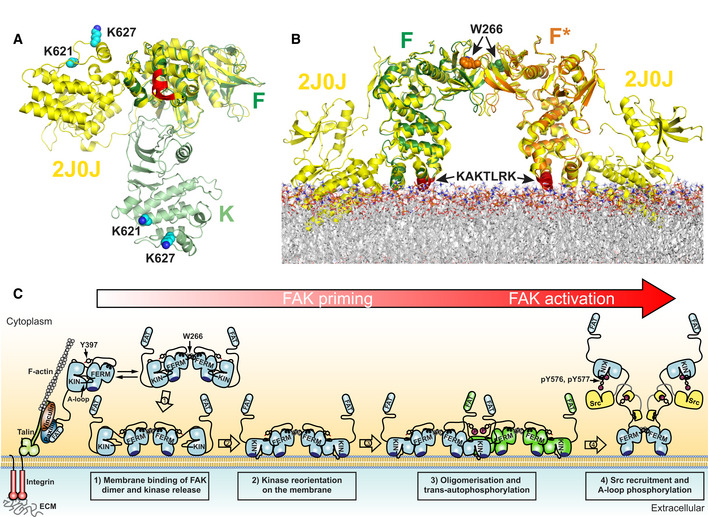
Schematic model for PI(4,5)P2 induced FAK activation in focal adhesions. Locally increased concentrations of FAK upon focal adhesion targeting promotes formation of FERM mediated FAK dimers. Binding of FAK dimers via their KAKTLRK basic region (dark blue) to PI(4,5)P2‐rich membranes in focal adhesions causes dissociation of the autoinhibitory interaction between the FERM and Kinase (KIN) domains (step 1). The released kinase domains reorient to interact via their own basic residues with the membrane (step 2). This aligns a large surface (F–K’, K–K’, and F’–K in Fig
2A) for formation of the symmetric dimer and exposes the autophosphorylation site for efficient trans‐autophosphorylation across the symmetric dimer (step 3). Src is recruited to the autophosphorylation site and phosphorylates Y576 and Y577 in the activation loop (A‐loop) of FAK to induce catalytic activation of FAK (step 4). Possibly an additional mechanism is involved in removing the kinase domain from the membrane to expose the Src phosphorylation sites, thereby potentiating the activating step 4 (see text).