Abstract
Introduction and hypothesis
Cervical cancer complicated by complete uterine prolapse is a rare clinical entity and uniform management recommendations have yet to be determined. The aim of the current review was to examine the effects of management patterns on survival outcomes in cervical cancer patients with complete uterine prolapse.
Methods
A systematic review of the literature was conducted using three public search engines. This included case reports with detailed descriptions of tumor characteristics, cancer management, and survival outcomes. Treatment patterns and tumor characteristics were correlated to survival outcomes.
Results
There were 78 patients with cervical cancer with complete uterine prolapse. Their mean age was 63.7 years. The median duration of prolapse was 147.9 months and 22.2 % of the patients experienced persistent/recurrent prolapse after cancer treatment. The mean tumor size was 8.9 cm and squamous cell carcinoma (83.9 %) was the most common histologic type. The majority of patients (56.2 %) had stage I cancer. Tumor characteristics were similar across the treatment patterns. Survival outcomes were more favorable with surgery-based treatment (48 patients) than with radiation-based treatment (30 patients): 5-year recurrence-free survival rate 72.0 % vs. 62.9 % (p=0.057), and 5-year disease-specific overall survival rate 77.0 % vs. 68.2 % (p=0.017). After controlling for age and stage, surgery-based therapy remained an independent prognostic factor for better disease-specific overall survival outcome (hazard ratio 0.32, 95 % confidence interval 0.11 – 0.94, adjusted p=0.039).
Conclusions
Although limited in study size, our results at least suggest that surgery-based treatment may have a positive effect on survival outcome in cervical cancer patients with complete uterine prolapse.
Keywords: Cervical cancer, Uterine prolapse, Survival, Surgery, Radiotherapy, Review
Introduction
Cervical cancer remains the most common gynecologic malignancy in the world [1]. In a 2008 worldwide statistics report, an estimated 529,800 new cases of cervical cancer were diagnosed with 275,100 deaths from this disease. Treatment of cervical cancer is based on the stage of disease, with typical treatment involving a combination of surgery, chemotherapy and radiotherapy (RT). Not infrequently, medical comorbidities affect the recommended treatment modality. One of the comorbidities that is challenging for practitioners is uterine prolapse. Treatment recommendations for cervical cancer in the setting of uterine prolapse have not been well established.
Uterine prolapse is not a rare condition in elderly women. Some degree of genital prolapse is observed in 40 – 60 % of parous woman [2]. It is estimated that women have an 11 % life-time risk of needing surgery due to prolapse or incontinence [3], and this mainly affects women above the age of 50 years. Generally, this percentage of women with symptomatic prolapse is thought to be underestimated given that many women do not seek medical attention or pursue surgical intervention. Pelvic organ prolapse is associated with increasing age, parity, obesity, prior hysterectomy, repetitive increase in intraabdominal pressure, and connective tissue disorders [4]. There are varying degrees of uterine prolapse. Complete prolapse, or procidentia, is defined as herniation of the uterus with the entire vaginal vault.
Despite uterine prolapse being a common condition in women, the coexistence of cervical cancer and uterine prolapse is extremely rare. There is no reported incidence, but the available information suggests that the incidence of cervical cancer among those with procidentia is between 0.14 % and 1 % [5, 6]. In the literature, some authors have postulated that prolapse might be protective against cervical cancer while others have considered that the irritation of the cervix by being outside the body might increase the propensity for neoplastic changes [7–9]. However, no clear explanation has been validated.
Currently, no standard recommendations for treatment of this combined entity have been elucidated. Some speculate that surgical resection holds more benefit than RT and others argue the reverse; however, no data have been collected to show that one modality improves outcome more than the other [8, 10]. With a growing elderly population, one can assume that both procidentia and cervical cancer may become more prevalent and force physicians to develop optimal treatment plans. The current study was a systematic review of the literature with the aim of examining treatment patterns and their outcomes in the setting of cervical cancer complicated by complete uterine prolapse.
Materials and methods
Source and study selection
This review was conducted based on MOOSE guidelines for systematic review using PubMed, Ovid MEDLINE, and Web of Science to identify all reported cases of cervical cancer and complete uterine prolapse [11]. The following search terms were used to identify such cases: (1) “cervical cancer” “cervical carcinoma” “cancer of the cervix” or “carcinoma of the cervix” or “cervical neoplasm” or “neoplasm of the cervix” and (2) “prolapse” or “procidentia” or “pelvic organ prolapse” or “uterine prolapse”. In addition, a Medical Subject Headings (MeSH) search was performed in PubMed using the following terms: (1) “uterine cervical neoplasm” and (2) “prolapse” or “uterine prolapse” or “pelvic organ prolapse”. Inclusion criteria were defined as case reports or case series describing patients with cervical cancer and concurrent complete prolapse with detailed description of the cancer characteristics, cancer management, and survival outcomes in the English language. The references of each article were evaluated and references that met the inclusion criteria were included in this study. Exclusion criteria included review articles, original research, poor individual case description, unspecified prolapse, or preinvasive cervical disease. In addition, one case of cervical cancer with complete uterine prolapse at the author’s institution was added (supplemental method S1). The Institutional Review Board of our institution exempts review studies that examine published case reports.
Clinical information
From each of the individual cases, demographic information was obtained including age, gravity, parity; presenting symptoms; cancer characteristics including histologic type, largest tumor diameter, FIGO stage, and grade; prolapse description; treatment pattern of cervical cancer including type and timing of surgery, RT, and/or chemotherapy; and follow-up outcomes including recurrence and cause of death.
Definitions
When cancer stage was not documented, the FIGO 2009 system was used to assign the proper cancer stage based on the description of the physical examination. Carcinomatous change and type I carcinoma were defined as noninvasive carcinoma and therefore excluded. Complete uterine prolapse was defined as procidentia based on the previous criteria of Chen and Ng from 2007, as stage IV according to the qualitative and subjective Pelvic Organ Prolapse Quantification (POP-Q) system, as third degree according to the system of Beecham, as grade 4 according to the system of Baden and Walker, or if the cervix described outside the introitus [12–15]. Recurrence-free survival was defined as the time from the date of primary treatment to the date of documented first recurrence of disease. If there was no recurrence, recurrence-free survival was determined as the date of last follow-up. Disease-specific overall survival was defined as the between the date of primary treatment and the date of death related to cervical cancer or the last follow-up.
Statistical analysis
The primary outcome of interest was survival of cervical cancer patients based on the type of initial treatment (surgery or RT). Continuous variables were assessed for normality by the Kolmogorov–Smirnov test expressed as appropriate as mean (±SD) or median (range). Student’s t test or the Mann–Whitney U test was performed for continuous variables as appropriate. Categorical variables were evaluated using Fisher’s exact test or the chi-squared test as appropriate, and expressed in terms of odds ratio (OR) and 95 % confidence interval (CI). To determine the significance of the correlation between variables and the survival outcomes recurrence-free survival and disease-specific overall survival, univariate (log-rank test) and multivariate (Cox proportional hazards regression test) analyses were performed as appropriate, and expressed in terms of hazard ratio (HR) and 95 % CI. Survival curves were constructed using Kaplan-Meier methodology. P<0.05 was considered statistically significant (all two-tailed). Statistical Package for the Social Sciences (SPSS, version 12.0, IL) was used for all analyses.
Results
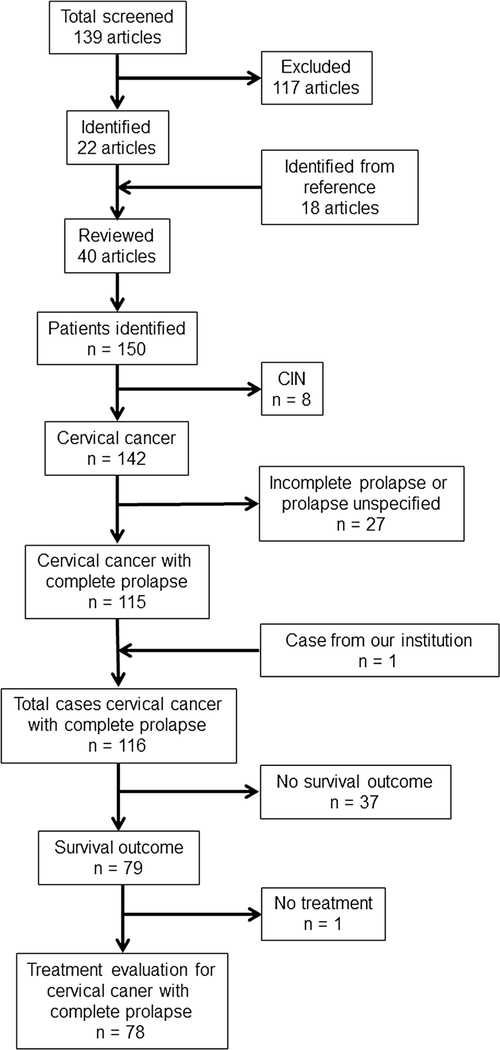
Selection of articles and identification of patients are shown in Fig. 1. A total of 139 articles were obtained from the search engines mentioned above. Review of the article abstracts produced 22 articles that met the inclusion criteria. A comprehensive review of the cited sources of the articles produced an additional 18 articles. From these 40 articles, 142 individual patients with invasive cervical cancer were identified. Of the 142 patients with invasive cervical cancer, 115 had a description of prolapse that met this study’s definition of “complete uterine prolapse” making a total of 115 patients with invasive cervical cancer and complete uterine prolapse who met the criteria. Among these, there were 78 patients who underwent treatment for cervical cancer with information available on survival outcome that could be further evaluated for statistical analysis (21 articles) [5, 7–9, 16–32].
Fig. 1.
Selection of articles and identification of patients with cervical cancer and complete uterine prolapse. PubMed, Ovid MEDLINE, and Web of Science were used for systematic literature search. CIN cervical intraepithelial neoplasia
Patient demographics are shown in Table 1. The majority of cases were published in 2000 or earlier (91.0 %) and reported in North America (56.4 %). The mean patient age was 63.7 years, and a large proportion of the patients were multiparous (84.2 %). Vaginal bleeding (53.8 %) and vaginal discharge (21.8 %) were the two most common presenting symptoms. There were 9 patients (11.5 %) with cervical cancer incidentally diagnosed at the time of hysterectomy for the indication of prolapse. Among 67 patients who had information available regarding recurrent status, recurrence was reported in 12 (17.9 %). The median follow-up time was 24 months in this study. Cervical cancer-related death was reported in 19 patients (24.4 %), and there were 10 patients (12.8 %) who died of other diseases.
Table 1.
Demographics of the patients with concurrent cervical cancer and prolapse
| Demographic variable | Valuee |
|---|---|
| No. of patients | 78 |
| Year of case publication | |
| Before 2000 | 71 (91.0 %) |
| 2000 or later | 7 (9.0 %) |
| Area of case publication | |
| North America | 44 (56.4 %) |
| Europe | 18 (23.1 %) |
| South America | 11 (14.1 %) |
| Asia | 4 (5.1 %) |
| Africa | 1 (1.3 %) |
| Age of patients (years) | |
| Mean (±SD) | 63.7 (±13.3) |
| <70 | 45 (62.5 %) |
| ≥70 | 27 (37.5 %) |
| Multiparity | |
| No | 9 (15.8 %) |
| Yes | 48 (84.2 %) |
| Presenting symptomsa | |
| Vaginal bleeding | 42 (53.8 %) |
| Vaginal discharge | 17 (21.8 %) |
| Incidentalb | 9 (11.5 %) |
| Pelvic pain | 7 (9.0 %) |
| Urinary symptoms | 7 (9.0 %) |
| Weight loss | 5 (6.4 %) |
| Cancer recurrencec | |
| No | 55 (82.1 %) |
| Yes | 12 (17.9 %) |
| Cause of death | |
| Not stated | 49 (62.8 %) |
| Cervical cancer | 19 (24.4 %) |
| Other disease | 10 (12.8 %) |
Symptoms other than genital prolapse
Cervical cancer diagnosed incidentally in hysterectomy specimens
11 patients missing
Values are number (%), except mean (±SD) age; total number may not be 78 due to missing variables
The characteristics of complete uterine prolapse in the patients with concurrent cervical cancer are shown in Table 2. The mean duration of prolapse at the time of cervical cancer diagnosis was 147.9 months and the duration in more than half of the reported patients was 120 months or more. Pessary use for prolapse was rare (7.7 %). The characteristics of cervical cancer in the patients with concurrent uterine prolapse are shown Table 3. The mean tumor size at the time of diagnosis was 8.9 cm and the majority had a bulky cervix tumor (68.8 %) and squamous cell histology (83.9 %). Over half of the patients (56.2 %) had stage I disease and about one-fourth (27.4 %) had stage II disease.
Table 2.
Characteristics of uterine prolapse in the 78 patients with cervical cancer
| Characteristic | Valuea |
|---|---|
| Duration (months) | |
| Mean (±SD) | 147.9 (±120.8) |
| <120 | 19 (43.2 %) |
| ≥120 | 25 (56.8 %) |
| Pessary use | |
| No | 72 (92.3 %) |
| Yes | 6 (7.7 %) |
| Recurrent prolapse | |
| No | 14 (77.8 %) |
| Yes | 4 (22.2 %) |
Values are number (%), except mean (±SD) duration; total number may not be 78 due to missing data
Table 3.
Characteristics of cervical cancer in the 78 patients with concurrent uterine prolapse
| Characteristic | Valueb |
|---|---|
| Tumor size (cm) | |
| Mean (±SD) | 8.9 (±5.7) |
| ≤4 | 5 (31.3 %) |
| >4 | 11 (68.8 %) |
| Histology type | |
| Squamous cell carcinoma | 52 (83.9 %) |
| Adenocarcinomaa | 4 (6.5 %) |
| Basal cell carcinoma | 2 (3.2 %) |
| Transitional cell carcinoma | 2 (3.2 %) |
| Others | 2 (3.2 %) |
| Stage (detailed) | |
| I (NOS) | 22 (28.2 %) |
| IB (NOS) | 11 (14.1 %) |
| IB1 | 4 (5.1 %) |
| IB2 | 4 (5.1 %) |
| II (NOS) | 10 (12.8 %) |
| IIA | 7 (9.0 %) |
| IIA2 | 2 (2.6 %) |
| IIB | 1 (1.3 %) |
| III (NOS) | 7 (9.0 %) |
| IIIA | 1 (1.3 %) |
| IIIB | 2 (2.6 %) |
| IVA | 2 (2.6 %) |
| Stage (ordinal) | |
| I | 41 (56.2 %) |
| II | 20 (27.4 %) |
| III | 10 (13.7 %) |
| IV | 2 (2.7 %) |
NOS not otherwise specified
includes one patient with adenosquamous histology
Values are number (%), except mean (±SD) tumor size; total number may not be 78 due to missing data
Treatment patterns of cervical cancer are shown in Table 4. The most common treatment type was RT alone (38.5 %) followed by surgery alone (33.3 %). Adjuvant hysterectomy after RT was seen in 11.5 % of patients, while adjuvant RT after surgical treatment was seen in 16.7 % of patients. Whole pelvic RT with intracavitary brachytherapy was the most common treatment type in both the definitive treatment setting (53.3 %) and in patients in whom hysterectomy was performed after RT (55.6 %). Conversely, whole-pelvis RT alone was the most common type of RT prescribed after hysterectomy (46.2 %). Among patients who underwent surgical treatment alone, vaginal hysterectomy was the most common hysterectomy type (38.5 %) followed by vaginal radical hysterectomy (30.8 %). Similarly, when surgery was performed in the adjuvant setting after RT, vaginal hysterectomy was the most common type of hysterectomy (55.6 %) followed by vaginal radical hysterectomy (22.2 %). Conversely, vaginal radical hysterectomy was the most common hysterectomy type among patients who underwent surgery followed by adjuvant RT (53.8 %). Pelvic lymphadenectomy was infrequently performed in the study population in which the majority underwent vaginal surgery (14.6 %).
Table 4.
Treatment patterns in patients with cervical cancer complicated by uterine prolapse
| Treatment pattern | Treatment | No. (%) of patients |
|---|---|---|
| RT alone 30 (38.5) | ||
| Type of RT | WPRT+ICBT | 16 (53.3) |
| WPRT alone | 7 (23.3) | |
| ICBT alone | 7 (23.3) | |
| RT followed by surgery 9 (11.5) | ||
| Type of RT | WPRT+ICBT | 5 (55.6) |
| WPRT alone | 2 (22.2) | |
| ICBT alone | 2 (22.2) | |
| Type of surgery | VH | 5 (55.6) |
| VRH | 2 (22.2) | |
| RH (NOS) | 1 (11.1) | |
| Hysterectomy (NOS) | 1 (11.1) | |
| Surgery alone 26 (33.3) | ||
| Type of surgery | VH | 10 (38.5) |
| VRH | 8 (30.8) | |
| Cervical amputation | 4 (15.4) | |
| Pelvic exenteration | 2 (7.7) | |
| Abdominal RH | 1 (3.8) | |
| Surgery followed by RT 13 (16.7) | ||
| Type of surgery | Vaginal RH | 7 (53.8) |
| Cervical amputation | 2 (15.4) | |
| TAH | 1 (7.7) | |
| TLRH | 1 (7.7) | |
| VH | 1 (7.7) | |
| Hysterectomy (NOS) | 1 (7.7) | |
| Type of RT | WPRT+ICBT | 2 (15.4) |
| WPRT alone | 6 (46.2) | |
| ICBT alone | 5 (38.5) | |
| Other surgical procedure | Pelvic LND | 7 (14.6) |
| Prolapse repair | 9 (18.8) | |
| Mesh placement | 1 (2.1) | |
RT radiotherapy, WPRT whole-pelvis radiotherapy, ICBT intracavitary brachytherapy, VH vaginal hysterectomy, VRH vaginal radical hysterectomy, RH radical hysterectomy, NOS not otherwise specified, TAH total abdominal hysterectomy, TLRH total laparoscopic radical hysterectomy, LND lymphadenectomy
Correlations between clinical characteristics of the patients and treatment modalities are presented (Table 5). Comparing the variables across the four treatment groups (RT alone, RT+surgery, surgery alone, surgery+RT), there were no significant differences in age (p=0.32), area of publication (p=0.061), year of publication (p=0.094), previous duration of prolapse (p=0.55), tumor size (p=0.31), and FIGO stage (p=0.86).
Table 5.
Clinical demographics and patterns of cervical cancer treatment
| RT alone | RT+surgery | Surgery alone | Surgery+RT | p valuea | |
|---|---|---|---|---|---|
| Age (years) | |||||
| <70 | 19 (63.3 %) | 6 (66.7 %) | 12 (52.1 %) | 8 (88.9 %) | 0.32 |
| ≥70 | 11 (36.7 %) | 3 (33.3 %) | 11 (47.9 %) | 1 (11.1 %) | |
| Area of case publication | |||||
| North America | 22 (73.3 %) | 5 (55.6 %) | 10 (38.5 %) | 6 (46.2 %) | 0.061 |
| Other areas | 8 (26.7 %) | 4 (44.4 %) | 16 (61.5 %) | 7 (53.8 %) | |
| Year of case publication | |||||
| Before2000 | 30 (100 %) | 8 (88.9 %) | 23 (88.5 %) | 10 (76.9 %) | 0.094 |
| 2000 or later | 0 | 1 (11.1 %) | 3 (11.5 %) | 3 (23.1 %) | |
| Duration of prolapse (months) | |||||
| <120 | 11 (55.0 %) | 2 (50 %) | 5 (31.3 %) | 2 (40 %) | 0.55 |
| ≥120 | 9 (45.0 %) | 2 (50 %) | 11 (68.8 %) | 3 (60 %) | |
| Histology | |||||
| SCC | 22 (88 %) | 6 (100 %) | 15 (78.9 %) | 9 (75 %) | 0.47 |
| Non-SCC | 3 (12 %) | 0 | 4 (21.1 %) | 3 (25 %) | |
| Tumor size (cm) | 0.31 | ||||
| ≤4 | 0 | 0 | 4 (50 %) | 1 (33.3 %) | |
| >4 | 4 (100 %) | 1 (100 %) | 4 (50 %) | 2 (66.7 %) | |
| Stage | 0.86 | ||||
| I | 15 (51.7 %) | 4 (50 %) | 15 (62.5 %) | 7 (58.3 %) | |
| II – IV | 14 (48.3 %) | 4 (50 %) | 9 (37.5 %) | 5 (41.7 %) | |
Chi-squared test
RT radiotherapy, SCC squamous cell carcinoma.
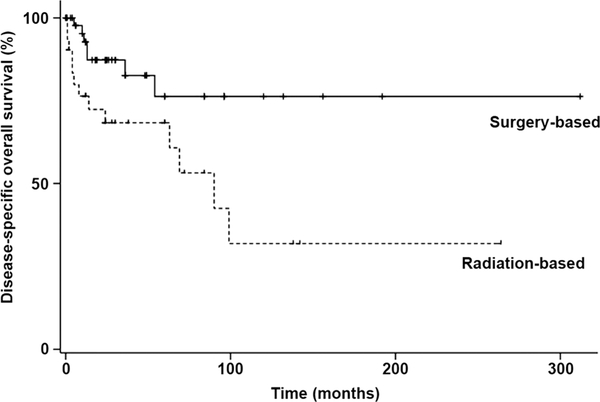
Survival outcomes in the patients with cervical cancer and complete uterine prolapse are shown in Table 6. Surgery-based treatment (surgery alone, surgery+RT, and RT+surgery; 48 patients) was associated with marginally but significantly better recurrence-free survival than radiation-based treatment (RT alone; 30 patients): 5-year rate 72.0 % and 62.9 %, respectively (p=0.057). Surgery-based treatment was associated with significantly better disease-specific overall survival than radiation-based treatment: 5-year rate 77.0 % and 68.2 %, respectively (p=0.017; Fig. 2). In multivariate analysis controlling for age and stage, surgery-based treatment remained an independent prognostic factor for better disease-specific overall survival than radiation-based treatment (HR 0.32, 95 % CI 0.11 – 0.94, adjusted p=0.039; Table 7). In patients treated with RT alone, RT+surgery, surgery alone, and surgery+RT, the 5-year recurrence-free survival rates were 85.1 %, 83.3 %, 81.4 %, and 90.5 %, respectively, and the 5-year disease-specific overall survival rates were 70.6 %, 86.7 %, 85.6 %, and 34.5 %, respectively (Fig. S1). Advanced stage remained an independent predictor of decreased survival of cervical cancer patients with complete uterine prolapse (recurrence-free survival, HR 5.53, 95 % CI 1.99 – 15.4, p<0.01; disease-specific overall survival, HR 5.91, 95 % CI 1.92 – 18.2, p<0.01; Table 7).
Table 6.
Survival analysis in patients with cervical cancer complicated by complete uterine prolapse
| Variable | No. of patients at risk | Recurrence-free survival |
Disease-specific overall survival |
||||
|---|---|---|---|---|---|---|---|
| 5-year rate (%) | HR (95 % CI) | p valuea | 5-year rate (%) | HR (95 % CI) | p valuea | ||
| Age (years) | |||||||
| <70 | 45 | 72.4 | 1 | 0.96 | 74.1 | 1 | 0.89 |
| ≥70 | 27 | 59.1 | 1.03 (0.41 – 2.57) | 84.0 | 1.07 (0.40 – 2.90) | ||
| Year of case publication | |||||||
| Before 2000 | 71 | 82.4 | 1 | 0.45 | 73.5 | 1 | 0.32 |
| 2000 or later | 7 | 100 | na | 100 | na | ||
| Duration of prolapse (months) | |||||||
| <120 | 19 | 75.5 | 1 | 0.51 | 72.5 | 1 | 0.28 |
| ≥120 | 25 | 94.7 | 0.57 (0.10 – 3.12) | 81.8 | 0.53 (0.16 – 1.76) | ||
| Histology | |||||||
| SCC | 52 | 79.8 | 1 | 0.68 | 65.6 | 1 | 0.56 |
| Non-SCC | 10 | 100 | 0.65 (0.08 – 5.22) | 88.9 | 0.65 (0.15 – 2.83) | ||
| Tumor size (cm) | |||||||
| ≤4 | 5 | na | 1 | 0.54 | na | 1 | 0.45 |
| >4 | 11 | 87.5 | na | 77.8 | na | ||
| Stage | |||||||
| I | 41 | 89.8 | 1 | 0.53 | 79.3 | 1 | 0.14 |
| II – IV | 32 | 68.7 | 1.45 (0.45 – 4.75) | 72.4 | 2.02 (0.77 – 5.28) | ||
| Treatment pattern | |||||||
| RT-basedb | 30 | 62.9 | 1 | 0.057 | 68.2 | 1 | 0.017 |
| Surgery-basedc | 48 | 72.0 | 0.46 (0.21 – 1.05) | 77.0 | 0.34 (0.14 – 0.87) | ||
SCC squamous cell carcinoma, na not available
Univariate analysis with log-rank test
RT alone
Surgery alone, surgery+RT, and RT+surgery
Fig. 2.
Disease-specific overall survival of cervical cancer patients with complete uterine prolapse in relation to treatment (surgery versus RT). Log-rank test for p value 0.017). Dashed line RT alone (30 patients), black line surgery alone, surgery followed by RT, and surgery after RT (48 patients)
Table 7.
Multivariate analysis for survival outcome: surgery versus RT
| Variable | No. of patients at risk | Recurrence-free survival |
Disease-specific overall survival |
||||
|---|---|---|---|---|---|---|---|
| 5-year rate (%) | HR (95 % CI) | p valuea | 5-year rate (%) | HR (95 % CI) | p valuea | ||
| Age (years) | |||||||
| <70 | 45 | 72.4 | 1 | 0.56 | 74.1 | 1 | 0.61 |
| ≥70 | 27 | 59.1 | 0.74 (0.27 – 2.04) | 84.0 | 0.75 (0.25 – 2.26) | ||
| Stage | |||||||
| I | 41 | 89.8 | 1 | <0.01 | 79.3 | 1 | <0.01 |
| II – IV | 32 | 68.7 | 5.53 (1.99 – 15.4) | 72.4 | 5.91 (1.92 – 18.2) | ||
| Treatment pattern | |||||||
| Radiation-basedb | 30 | 62.9 | 1 | 0.14 | 70.6 | 1 | 0.039 |
| Surgery-basedc | 48 | 72.0 | 0.50 (0.20 – 1.26) | 86.7 | 0.32 (0.11 – 0.94) | ||
A Cox proportional hazards regression model with age, stage and treatment pattern entered for adjusted p values
RT alone
Surgery alone, surgery+RT, and RT+surgery
Treatment patterns and outcomes in patients with pelvic organ prolapse were examined. Detailed descriptions of the pelvic organ prolapse were rarely reported in these 78 patients. There were only 10 patients (12.8 %) in whom surgical repair was performed, 9 of these 10 patients underwent concurrent prolapse repair with surgical treatment for the cervical cancer, representing 18.8 % of 48 surgical cases (surgery alone, surgery+RT, and RT+surgery). One patient died related to large blood loss during cervical cancer surgery and no details of pelvic organ prolapse repair were provided. Among the 9 patients, anterior colporrhaphy was the most common type of prolapse repair (55.6 %) followed by perineorrhaphy (33.3 %), posterior repair (11.1 %), and colpocleisis (11.1 %). Mesh placement was only used in 1 patient (2.2 %) among the 48 patients treated surgically. The mesh used was polyethylene sheet, and the patient did not undergo RT before or after cervical cancer surgery; no follow-up information for the prolapse was available.
Information regarding the status of prolapse after the cancer treatment was provided for 18 (23.1 %) of the 78 patients, and was not provided for the remaining 60 patients (76.9 %). Among the 18 patients, prolapse was reported in four (22.2 %, 95 % CI 3.0 – 41.4), and in three of these patients prolapse persisted after RT and in one prolapse recurred after surgical treatment. Additionally, in two patients treated surgically for both prolapse and cervical cancer, no recurrence of prolapse was reported, and of these one had an anterior repair, one had a repair with vaginal hysterectomy, and the other had colpocleisis after neoadjuvant chemoradiotherapy. In two patients no recurrence of prolapse was reported after surgical treatment of the cervical cancer without specific prolapse-directed surgery; both of these patients underwent vaginal radical hysterectomy.
Discussion
The respective treatment strategies for cervical cancer and pelvic organ prolapse are well established. However, the coexistence of cervical cancer and pelvic organ prolapse is extremely rare and the best treatment is unclear [27]. The current study was a systematic review of the literature with the aim of examining the treatment strategies and outcomes of cervical cancer complicated by complete uterine prolapse. The results suggest that the prognosis following surgery-based treatment of cervical cancer patients with complete uterine prolapse may be favorable, and thus the recommended treatment option should be based on the surgeon’s preference, patient and tumor factors, and consideration of medical comorbidities.
The rarity of cervical cancer in the setting of complete uterine prolapse leaves practitioners with limited reference on which to base treatment decisions. Difficulties surface in the assessment and treatment of carcinoma arising in patients with complete uterine prolapse. Challenges include the difficulty in clinical staging because of the inability to reduce the prolapsed uterus or preexisting ulcerations. In addition, prolapse and peritumor edema may distort the anatomy impairing surgical and RT planning whilst a patient’s complaint of pelvic organ prolapse may require surgical intervention for relief.
There are a number of hypotheses with regard to the pathophysiology of concurrent cervical cancer and pelvic organ prolapse. Some authors have speculated that prolapse might be protective against cervical cancer while others have concluded the opposite [7–9]. Edwards and Beebe. in a case report of cervical cancer in association with procidentia, postulated that the cornification of the epithelium in prolapse of the uterus may confer resistance on the cervical tissues to the development of carcinoma [5]. Lewis et al. also suggested that the resulting cornification may be protective [33]. The authors also commented that free drainage from the prolapsed organ may remove the constantly present irritating acidic secretions and that the acid environment itself may predispose the patient to cancer development. Emmert et al. postulated that occlusion of lymphatic drainage due to anatomic changes such as parametria stretching may prevent the dissemination of cancer cells [34]. In our literature review, in the vast majority of cervical cancer patients with complete uterine prolapse the disease was early stage (stage I 56.2 %). Because cervical cancer patients in the most of the world present with locally advanced disease, our results may partly support the hypothesis of the protective effect of uterine prolapse, although cervical cancer detected because of concurrent uterine prolapse may be more likely to be early stage disease.
In contrast, others have hypothesized that the irritation of the cervix by being outside the body may encourage cancer progression. For instance, Emmert et al. suggested that excessive cornification of prolapse could favor the development of cancer [34]. de Lima also commented that lymphatic occlusion is unlikely to be protective against cervical cancer development in the prolapsed uterus because cancer of the vulva usually develops in poorly nourished sclerotic tissues [10]. Other controlling factors have been hypothesized to be important in the development of cancer in this patient population including age [34], duration of the prolapse [28], and pessary use [27]. Rocker suggested that procidentia of more than 10 years increases the risk of developing neoplastic changes in the prolapse [28]. Furthermore, Reimer et al. suggested that pessary use is predisposes to cancer development due to foreign body-related chronic inflammation [27]. Our results do not answer the question as to whether or not the presence of chronic inflammation secondary to complete uterine prolapse triggers an oncogenic pathway. Because stage-specific survival outcomes in cervical cancer patients with complete uterine prolapse (5-year recurrence-free survival: stage I 89.8 % vs. stage II – IV 68.7 %) seems not inferior to that in cervical cancer patients without complete uterine prolapse [35], it can at least be speculated that complete uterine prolapse may not have adverse effects on progression of cervical cancer.
The curative treatment options for cervical cancer in the setting of uterine prolapse generally include surgery or RT alone, or a combination of the two treatments, that is, either surgery followed by RT, or RT followed by surgery. RT alone (38.5 %) and surgery alone (33.3 %) were the two most common treatment patterns in our study. Some authors have recommended certain interventions over others. For example, Cabaniss and Crocker suggested that surgical intervention is the treatment of choice because of the benefits of providing symptom relief and avoiding the danger of visceral injury by ionizing radiation [17]. Our results showed a possible impact of surgery-based approaches on prognosis in cervical cancer patients with complete uterine prolapse. However, given the retrospective nature of the study, the exact indications for treatment allocation were not able to adjust for the analysis, and therefore, the results need to be interpreted with caution for possible missing confounders. Further studies with a prospective design are warranted.
Among the surgical interventions in this review, the most common was vaginal hysterectomy (in 33 patients out of 48 treated surgically, 68.8 %). This finding is unique to cervical cancer patients with complete uterine prolapse because the common surgical modality for cervical cancer without prolapse is the abdominal approach. It is speculated that the presence of complete uterine prolapse enables surgeons to access the surgical site more easily with a vaginal approach. In the studies highlighting vaginal hysterectomy as the treatment of choice, older patients were spared the morbidity of an open abdominal procedure. Often, severe comorbidities, especially in geriatric patients, and distorted anatomy make radical surgery difficult [27]. Ultimately, in most reports, later stage disease and poor health precluded patients from undergoing surgery.
RT is equivalent to surgery in early-stage cervical cancer. In this review, the most common treatment was whole pelvic RT with intracavitary brachytherapy. In all reported cases, reduction of genital prolapse prior to RT was highly recommended in order to decrease visceral injury and the formation of a vesicovaginal or rectovaginal fistula from the ionizing radiation [26, 27]. However, Reimer et al. reported a case of cervical cancer with irreducible procidentia [27]. This patient was treated with pelvic external irradiation at a total dose of 52.2 Gy with concomitant weekly cisplatin. The patient’s radiation portal was adjusted by microbeam RT-based planning to minimize radiation-induced injury. Harvey and Ritchie described another patients with irreducible prolapse treated with RT [36]. They used a flat lead rubber shield cut to fit around the extruded uterine side that was placed against the perineum to protect the bladder, rectum and labia. Of note, Graham et al. reported that, in addition to reduction of the prolapsed uterus during RT, a decrease in radiation dose may be important due to lymphatic and venous stasis interfering with healing [34].In this setting, intensity-modulated RT may be an option for the treatment of cervical cancer with complete prolapse [34].
In some but not all patients, the prolapse resolved after RT because of the presumed fibrosis effect [37]. Emergent RT can be provided to those with incarcerated prolapse complicated by a hemorrhaging cervical tumor. One consideration is to offer neoadjuvant RT in order to ease subsequent surgery and prevent unforeseen complications by reducing the tumor mass. Moreover, prior administration of RT may reduce the extent and risk of postoperative complications. In circumstances that do not allow conventional surgical treatment or RT, palliation with vaginal anterior exenteration has been reported. This procedure seems to be reserved for patients, usually those of advanced aged, who are extremely symptomatic and in whom abdominal surgery is risky [37]. Among the 48 patients who underwent cervical cancer surgery, there was only one (2.2 %) who underwent mesh placement. Synthetic mesh was used and the patient did not receive any RT after mesh placement. There remains a lack of evidence to support the use of mesh in the setting of RT for gynecologic malignancy, and further studies are warranted.
A strength of this study is that it involved a comprehensive literature review for a rare cervical cancer complication in an under-studied clinical entity. Despite the relatively large sample size in this study, the numerous differing treatment approaches made the analysis difficult (type II error). The high variability in treatment approaches was possibly related to the lack of universal treatment recommendations. A weakness of the study is the retrospective design. For instance, there were only 18 cases (23.1 %) for which information on follow-up of the pelvic organ prolapse was specifically provided. We do not know if in the remaining 60 cases, the patients had their prolapse reduced and if there was any evidence of recurrence either symptomatically or objectively. The low reporting rate may be due to the relatively short follow-up time of 24 months. Pelvic organ prolapse could recur later than 24 months after surgery; therefore long-term follow-up could be required. Another notable limitation of the current study was the limited information about the decision-making process during treatment planning. Furthermore, surgical complications and adverse effects of radiation were rarely reported in the literature reviewed for this project.
In summary, this review found that survival outcomes of cervical cancer patients with complete uterine prolapse are more favorable with surgery-based treatment than with radiation-based treatment, although the study population was of limited size. While additional accumulating evidence and validation by others is needed, it is at least suggested that a surgical approach needs to be considered in women with cervical cancer complicated by complete uterine prolapse if the patient’s condition and medical comorbidity are not limitations to surgery.
Supplementary Material
Acknowledgments
The authors thank Drs. Katherine E. Tierney, MD, Kodama Michiko, MD, PhD, and Mikio Mikami, MD, PhD, for scientific input and technical support for this study.
Footnotes
Conflicts of interest None.
Electronic supplementary material The online version of this article (doi:10.1007/s00192-015-2731-8) contains supplementary material, which is available to authorized users.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90 [DOI] [PubMed] [Google Scholar]
- 2.Maher C, Feiner B, Baessler K, Schmid C (2013) Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 4, CD004014. [DOI] [PubMed] [Google Scholar]
- 3.Richter HE, Varner ER (2007) Pelvic organ prolapse In: Berek JS (ed) Berek and Novak’s gynecology, vol 24 Lippincott Williams and Wilkins, Philadelphia, pp 897–934 [Google Scholar]
- 4.Swift S, Woodman P, O’Boyle A, Kahn M, Valley M, Bland D et al. (2005) Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol 192:795–806 [DOI] [PubMed] [Google Scholar]
- 5.Edwards EA, Beebe RA (1950) Carcinoma of the cervix in association with procidentia. Q Bull Northwest Univ Med Sch 24:12. [PMC free article] [PubMed] [Google Scholar]
- 6.Mehboob R, Ahmad N (2002) Unexpected pathology at vaginal hysterectomy for genital prolapse. Pak J Med Res 41:1–5 [Google Scholar]
- 7.Loizzi V, Cormio G, Selvaggi L, Carriero C, Putignano G (2010) Locally advanced cervical cancer associated with complete uterine prolapse. Eur J Cancer Care (Engl) 19:548–550 [DOI] [PubMed] [Google Scholar]
- 8.da Silva BB, da Costa AR, Filho CP, Melo JA (2002) Carcinoma of the cervix in association with uterine prolapse. Gynecol Oncol 84: 349–350 [DOI] [PubMed] [Google Scholar]
- 9.Cabrera S, Franco-Camps S, Garcia A, Verges R, Diaz-Feijoo B, Perez-Benavente MA et al. (2010) Total laparoscopic radical hysterectomy for cervical cancer in prolapsed uterus. Arch Gynecol Obstet 282:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lima OA (1952) Cervical cancer and prolapse of the uterus. Obstet Gynecol Surv 7:849–850 [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 12.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO et al. (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175: 10–17 [DOI] [PubMed] [Google Scholar]
- 13.Beecham CT (1980) Classification of vaginal relaxation. Am J Obstet Gynecol 136:957–958 [DOI] [PubMed] [Google Scholar]
- 14.Baden WF, Walker TA (1972) Genesis of the vaginal profile: a correlated classification of vaginal relaxation. Clin Obstet Gynecol 15:1048–1054 [DOI] [PubMed] [Google Scholar]
- 15.Chen GD, Ng CS (2007) Updated definition of female pelvic organ prolapse. Incont Pelvic Floor Dysfunct 1:121–124 [Google Scholar]
- 16.Ashton DL (1948) Carcinoma of the cervix complicating procidentia uteri. Am J Obstet Gynecol 55:299–302 [DOI] [PubMed] [Google Scholar]
- 17.Cabaniss CM, Crocker CL (1963) Carcinoma of the prolapsed cervical stump. Report of a case. Obstet Gynecol 22:606–9 [PubMed] [Google Scholar]
- 18.Calame RJ, Perticucci S, de Fazio L (1970) Multiple primary carcinoma of the uterus: a review. Obstet Gynecol Surv 25:191–206 [DOI] [PubMed] [Google Scholar]
- 19.Chambers CB (1975) Uterine prolapse with incarceration. Am J Obstet Gynecol 122:459–462 [DOI] [PubMed] [Google Scholar]
- 20.Dane B, Dane C, Erginbas M, Baran S, Cetin A (2009) Verrucous carcinoma of the cervix in a case with uterine prolapse. Ann Diagn Pathol 13:344–346 [DOI] [PubMed] [Google Scholar]
- 21.Daw E (1972) Carcinoma of cervix in association with procidentia. Br J Clin Pract 26:197–200 [PubMed] [Google Scholar]
- 22.Diaz-Bazan N (1964) Cervical carcinoma with procidentia in El Salvador: report of 10 cases with review of the literature. Obstet Gynecol 23:281–288 [PubMed] [Google Scholar]
- 23.Guthrie D, Bache W (1932) The Infrequency of carcinoma of the cervix with complete procidentia. Ann Surg 96:796–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hesselberg E (1963) Cancer of the cervix associated with procidentia. S Afr Med J 37:589–592 [PubMed] [Google Scholar]
- 25.Kistner RW (1957) Cervical carcinoma complicating procidentia; report of a case. Obstet Gynecol 10:482–6 [PubMed] [Google Scholar]
- 26.Kriplani A, Relan S, Kumar L, Biswal BM, Rath GK (1995) Incarcerated procidentia: a rare complication of carcinoma cervix. Aust N Z J Obstet Gynaecol 35:463–464 [DOI] [PubMed] [Google Scholar]
- 27.Reimer D, Sztankay A, Steppan I, Abfalter E, Lunzer H, Marth C et al. (2008) Cervical cancer associated with genital prolapse – a brief review of the literature and long-term results of successful treatment with radiochemotherapy and surgery in a very frail patient. Eur J Gynaecol Oncol 29:272–275 [PubMed] [Google Scholar]
- 28.Rocker I (1958) Malignant changes in procidentia; the incidence during the years 1949–54 at Addenbrooke’s Hospital, Cambridge. J Obstet Gynaecol Br Emp 65:89–91 [DOI] [PubMed] [Google Scholar]
- 29.Sholes DM Jr (1959) Carcinoma of the cervix and uterine prolapse. Obstet Gynecol 13:223–225 [DOI] [PubMed] [Google Scholar]
- 30.Stone BH, Mansell H (1955) Procidentia and cervical cancer. Obstet Gynecol 5:198–200 [PubMed] [Google Scholar]
- 31.Todd TF (1937) Two cases of carcinoma of the cervix complicating procidentia uteri. Proc R Soc Med (Section of Obstetrics and Gynaecology) 30:1343–1344 [PMC free article] [PubMed] [Google Scholar]
- 32.Wong WS, Ng CS, Lee CK (1990) Verrucous carcinoma of the cervix. Arch Gynecol Obstet 247:47–51 [DOI] [PubMed] [Google Scholar]
- 33.Graham JB, Sotto LSJ, Paloucek FP (1962) Carcinoma of the cervix, vol 12 WB Saunders Company, Philadelphia, pp 231–239 [Google Scholar]
- 34.Du XL, Tao J, Sheng XG, Lu CH, Yu H, Wang C et al. (2012) Intensity-modulated radiation therapy for advanced cervical cancer: a comparison of dosimetric and clinical outcomes with conventional radiotherapy. Gynecol Oncol 125:151–7 [DOI] [PubMed] [Google Scholar]
- 35.Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT et al. (2006) Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 95 (Suppl 1):S43–S103 [DOI] [PubMed] [Google Scholar]
- 36.Harvey RA, Ritchie RN (1943) Carcinoma of the cervix complicated by complete procidentia: radiation therapy. Radiology 41:48–51 [Google Scholar]
- 37.Way S (1958) Perineal anterior exenteration. Br J Obstet Gynaecol 65:24–27 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.