Fig. 1.
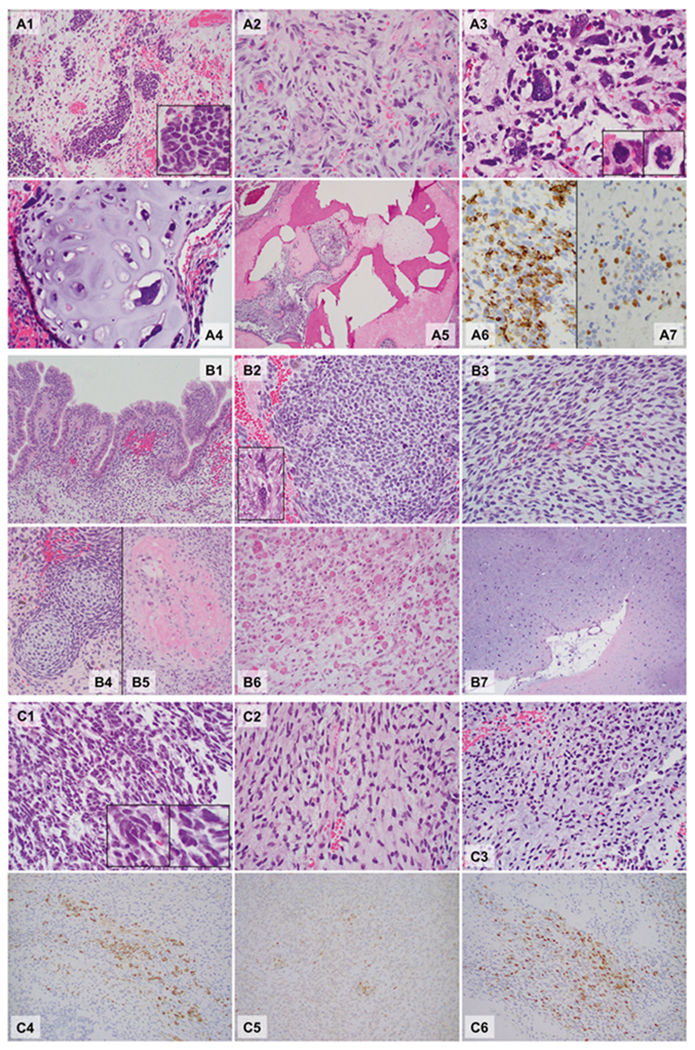
Microphotographs of the DICER1-associated ovarian sarcoma (a Patient 1), peritoneal sarcoma (b Patient 2), and primary intracranial sarcoma (c Patient 3). Hematoxylin and eosin sections, ×40: A5; ×200: A1, A4, B1, B4, B5, B6, and B7; ×400: A2, A3, B2, B3, C1, C2, and C3; ×400, Immunohistochemistry, ×400: A6, A7, C4, C5, and C6. Patient 1 (A1–A7): The tumor was ill-defined and comprised a heterogeneous population of loosely cohesive tumor cells demonstrating areas with undifferentiated small round blue cells (A1, box: high power), spindle cells (A2), large, bizarre pleomorphic cells with frequent mitoses (anaplasia; A3, box: mitoses) and islands of malignant cartilage scattered throughout the tumor (A4). Focal bone formation was present (A5). Immunohistochemistry showed that the undifferentiated small round blue cells and spindle cells were partially, strongly positive for desmin (A6), and a smaller number of cells were positive for myogenin (A7). Patient 2 (B1–B7): Peritoneal mass. The sections from the peritoneal mass contained cystic walls in variable thicknesses (Fig. 1B1–B5), lined by bland ciliated columnar cells (B1). The underlying stroma was expanded (overgrown) and demonstrated a heterogeneous population including undifferentiated small round blue cells (B2), spindle cells (B3), and scattered pleomorphic cells (anaplasia, B2 box). The small round blue cells formed a hypercellular zone, “cambium layer” (B1), immediately beneath the epithelium. Loose myxoid stroma with occasional islands of immature-appearing cartilage (B4) and focal osteoid formation (B5) were identified in the background. Autopsy (post chemotherapy, B6 and B7). The tumor was largely necrotic, but single and clusters of viable tumor cells were present throughout. The tumor cells demonstrated prominent rhabdomyoblastic differentiation with abundant eosinophilic cytoplasm and “strap cells” (B6). Pleomorphic cells were occasionally seen. The tumor also contained multiple broad areas of chondrosarcomatous differentiation with abundant chondroid matrix and occasional pleomorphic cells within the lacunae, which were occasionally multinucleated (B7). Patient 3 (C1–C6): The hematoxylin and eosin sections revealed a cellular, heterogenous population of tumor cells with occasional necrosis demonstrating areas with undifferentiated small round blue cells (C1) with scattered large pleomorphic cells (C1 box) and spindle cells (C2). Some areas show abundant myxoid to chondroid matrix in the background (C3). Immunohistochemistry staining showed that tumor cells were partially strongly positive for desmin (C4), MyoD1 (C5) and myogenin (C6). The immunoprofile is supportive of rhabdomyoblastic differentiation
