Fig. 4.
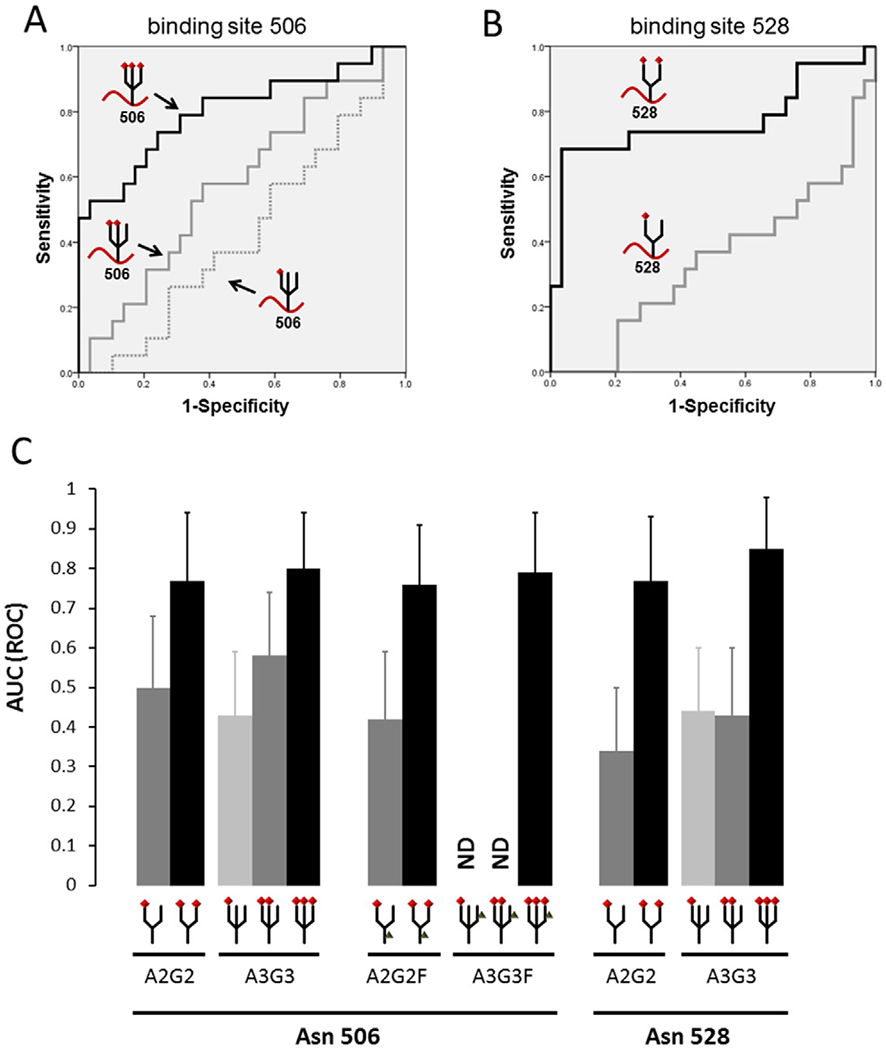
Comparison of fully versus partially sialylated alpha-chain of C4BP Area-under-curves (AUCs) of various glycans at the 506 and 528 sites of the C4BP alpha-chain in the serum of EOC (n = 19) and control subjects (healthy women n = 10, and endometrioma n = 19) were examined. (A) ROC curves of tri-antennary glycans on Asn 506 in the C4BP alpha-chain are shown. Fully-sialylated glycopeptides (binding 3 sialic acids) showed a significantly higher AUC than partially-sialylated glycans. (B) Receiver-operator-characteristic (ROC) curves of bi-antennary glycans on Asn 528 in the C4BP alpha-chain are shown. Fully-sialylated glycopeptides (binding 2 sialic acids) showed a markedly higher AUC than monosialylated glycans. (C) AUC values of each glycopeptide derived from the C4BP alpha-chain are shown. Comparing AUCs between epithelial ovarian cancer patients and the control group revealed that glycopeptides with fully-sialylated glycans showed significantly higher AUCs than those with partially-sialylated glycans. This trend was observed regardless of the binding sites and fucose binding. Error bars indicate 95% confidence interval of AUC. Red diamond-shape: sialic acids, Gray triangle-shape: fucose, A2G2: bi-antennary N-glycan, A3G3: tri-antennary N-glycan, A2G2F: bi-antennary N-glycan with one fucose, A3G3F: tri-antennary N-glycan with one fucose.
