Figure 1.
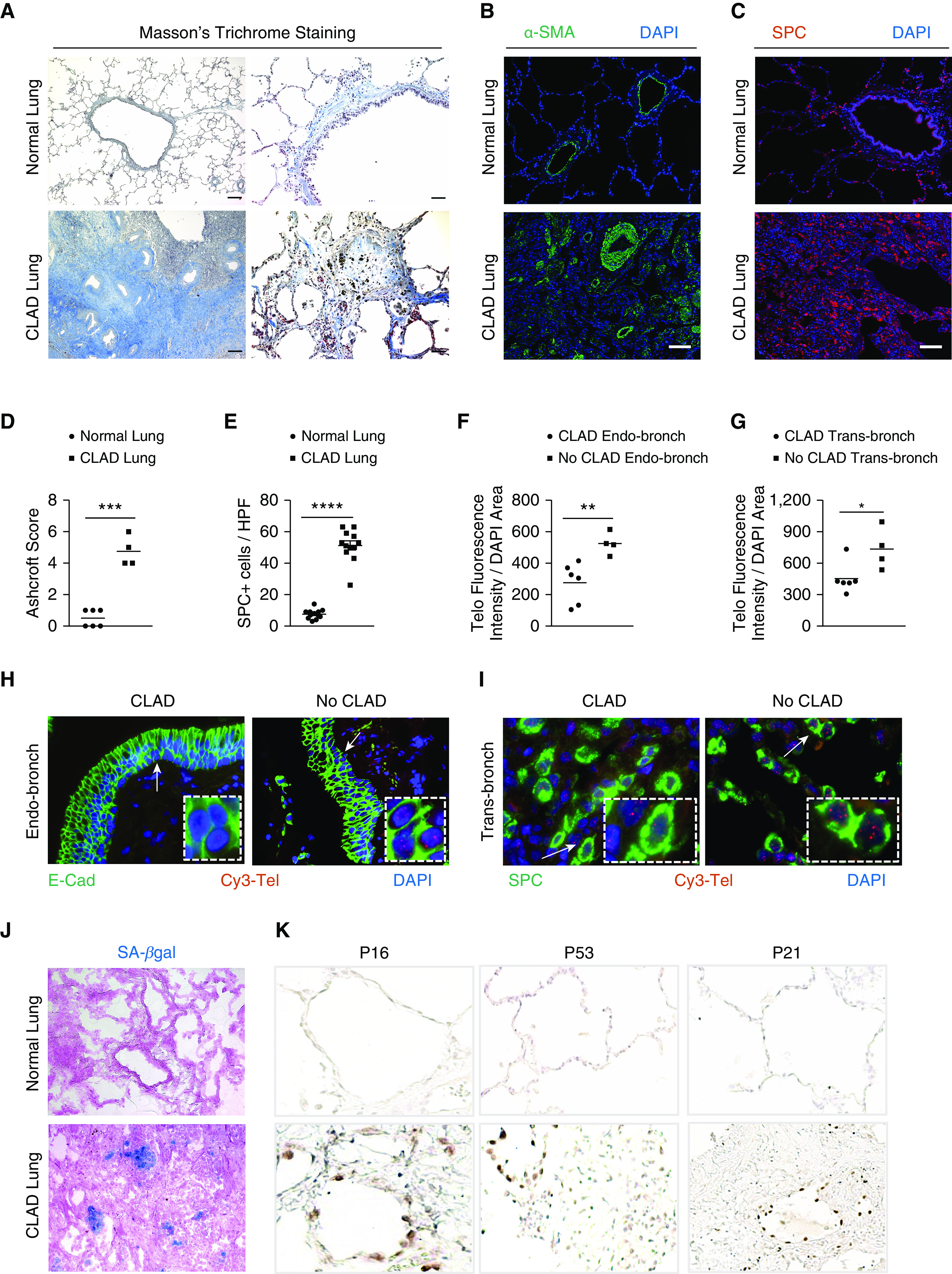
Chronic lung allograft dysfunction (CLAD) pathology in lung transplant recipients. (A) Masson’s trichrome staining in CLAD lungs and normal lungs. Collagen deposition (blue) is seen around the airways and in bronchiole, causing obliteration in CLAD lungs. Scale bars, 400 μm (left panels) and 100 μm (right panels). (B and C) Immunofluorescence staining on paraffin-embedded lung sections from normal and CLAD lungs with α-SMA antibody (B) and SPC antibody (C). Nuclei were stained with DAPI. N = 3. Scale bars, 100 μm. (D) Quantification of fibrosis using Ashcroft score. ***P < 0.001 (t test). (E) Quantification of the number of SPC immunoreactive cells per high-power field. Ten fields were used per group. ****P < 0.0001 (t test). (F and G) Quantification of telomere length by calculating telomere fluorescence intensity over the DAPI area in endobronchial and transbronchial biopsies diagnosed as CLAD and no CLAD. **P < 0.01 and *P < 0.05 (t test). (H and I) Representative images of telomere quantitative fluorescence in situ hybridization assay conducted on endobronchial and transbronchial biopsies diagnosed with CLAD and no CLAD. White arrows point to area depicted in the box. (J) Senescence-associated–β galactosidase staining (blue colored cells) on optimal cutting temperature–embedded cryosections counterstained with eosin. N = 6. (K) Immunohistochemistry on paraffin-embedded lung sections from normal and CLAD lungs with P16, P53, and P21 antibodies. N = 5. E-cad = E-cadherin; HPF = high-power field; SA-β-gal = senescence-associated–β galactosidase; SMA = smooth muscle actin; SPC = surfactant protein C.
