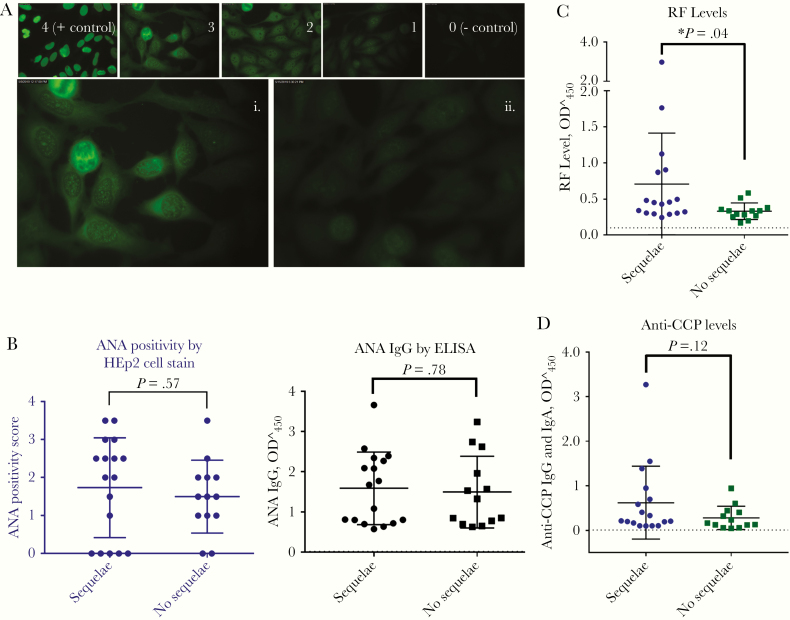
Figure 5.
A, Antinuclear antibody (ANA) immunoglobulin G (IgG) levels by human epithelial type 2 (HEp2) immunofluorescence assay, in survivors of Ebola virus (EBOV) disease with or without sequelae. Panels 0–4 represent examples of brightness scoring scale. Also shown are examples of survivors with (i) and without (ii) sequelae. B, ANA in EBOV survivors with (n = 17) or without (n = 13) sequelae, measured with both HEp2 cell immunofluorescence assay and enzyme-linked immunosorbent assay (ELISA) (P = .57 and .78, respectively, calculated with Student t test). C, Rheumatoid factor (RF) levels are significantly higher in EBOV survivors with sequelae than in those without sequelae (P = .04; Student t test). D, Anti–cyclic citrullinated peptide antibody (anti-CCP) antibody levels in EBOV survivors with or without sequelae, measured with ELISA (P = .12; Student t test). Dotted lines in B–D indicate negative control levels, as provided by the test kits. Abbreviation: OD450, optical density at 450 nm.