Figure 4.
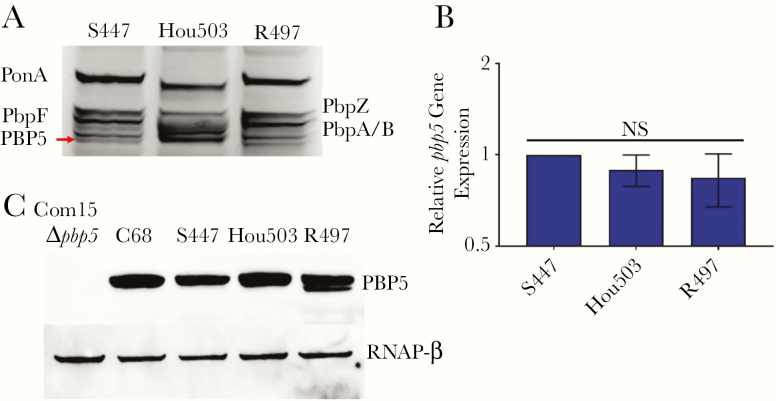
Penicillin-binding protein (PBP) 5 (PBP5) of HOU503 has increased β-lactam binding affinity, independent of gene expression and protein levels. A, PBP β-lactam affinity determined by Bocillin FL labeling of PBPs embedded in enterococcal membrane fractions, followed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualization of fluorescence intensities. PBP5 (red arrow) has increased band intensity in HOU503 relative to S447 and R497. B, There are no significant differences in pbp5 relative gene expression, as determined with quantitative reverse-transcription. Statistics were calculated with data from 3 biological replicates and 3 internal technical replicates. Data represent means with standard deviations (error bars). C, PBP5 expression levels determined by Western blotting cell lysates. Com15, a clade B strain lacking pbp5, was used as a negative control. C68, a hospital-associated clade A1 strain used to purify PBP5 for antibody production, served as a positive control. An antibody against the RNA polymerase β subunit (RNAP-β) was used as the loading control.