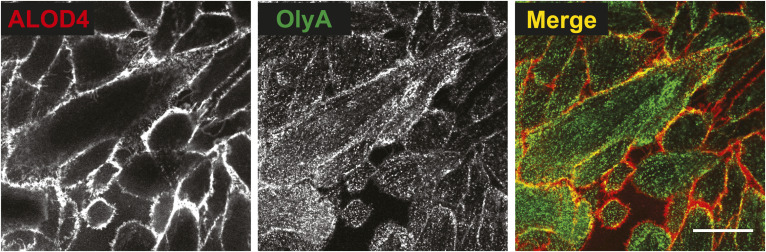
Cholesterol is the most abundant lipid in the plasma membranes (PMs) of animal cells. However, not all cholesterol molecules are available (or accessible) to participate in cellular signaling processes. Most of the PM cholesterol is sequestered by proteins and lipids such as SM. Cholesterol in excess of such sequestration is accessible at the cell surface and can be transported to the endoplasmic reticulum (ER) membrane, where it binds to cholesterol sensors and shuts down cholesterol synthesis and uptake (1, 2). Such control of the organization of cholesterol in PMs allows cells to maintain optimal cholesterol levels in the PM while avoiding cholesterol overaccumulation. The development of nonlytic probes for these distinct states of PM cholesterol have allowed for visualization and quantification of cholesterol accessibility (3, 4). In this image of CHO-K1 cells, accessible cholesterol is detected with ALOD4, a domain of anthrolysin O, a bacterial cholesterol-binding protein (red), and SM-sequestered cholesterol is detected with Ostreolysin A (OlyA), a fungal protein that binds SM/cholesterol complexes (green). Cells were set up in medium A at a density of 30,000 cells per well of a 35-mm MatTek dish with a #1.5 glass coverslip bottom. The next day, media was removed, cells were washed twice with PBS, then treated with medium A (500 µl onto the coverslip) containing ALOD4-594 (3 µM) and OlyA-488 (3 µM). After incubation for 10 min at room temperature, cells were washed twice with PBS, fresh medium A (1 ml) was added to the dish, and cells were imaged immediately with a Zeiss LSM 880 microscope in a live-cell chamber set to 37°C. The pinhole was set to 1 Airy unit (AU), and 488 nm and 561 nm lasers were used to excite OlyA-488 and ALOD4-594, respectively. ImageJ software was used to generate the grayscale and merge images (scale bar, 25 µm). While both ALOD4 and OlyA bind to the cell surface (peripheral staining), only OlyA is internalized into cells (speckled pattern) (4). This suggests that SM/cholesterol complexes may be enriched in areas of the PM that undergo endocytosis whereas accessible cholesterol is excluded from such areas. Instead, accessible cholesterol may rely on nonvesicular transporters, such as Aster/GramD1 proteins, to reach the ER and exert regulatory functions (5,6).
EQUIPMENT: Confocal Laser Scanning Microscope (Zeiss LSM 880; Instrument funded by NIH 1S10OD021684-01 to Kate Luby-Phelps)
REAGENTS: ALOD4-594 – ALOD4 labeled at its lone cysteine with Alexa Fluor 594 C5 maleimide (Invitrogen); OlyA-488 – OlyA labeled at its lone cysteine with Alexa Fluor 488 C5 maleimide (Invitrogen); Medium A – 1:1 mixture of Ham’s F-12 and DMEM supplemented with 5% FCS, 100 units/ml penicillin, and 100 µg/ml streptomycin sulfate.
REFERENCES
- 1.Das A., Brown M. S., Anderson D. D., Goldstein J. L., and Radhakrishnan A.. 2014. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife. 3: e02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infante R. E., and Radhakrishnan A.. 2017. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife. 6: e25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endapally S., Frias D., Grzemska M., Gay A., Tomchick D. R., and Radhakrishnan A.. 2019. Molecular discrimination between two conformations of sphingomyelin in plasma membranes. Cell. 176: 1040–1053.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson K. A., Endapally S., Vazquez D. C., Infante R. E., and Radhakrishnan A.. 2019. Ostreolysin A and anthrolysin O use different mechanisms to control movement of cholesterol from the plasma membrane to the endoplasmic reticulum. J. Biol. Chem. 294: 17289–17300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandhu J., Li S., Fairall L., Pfisterer S. G., Gurnett J. E., Xiao X., Weston T. A., Vashi D., Ferrari A., Orozco J. L. et al. . 2018. Aster proteins facilitate nonvesicular plasma membrane to ER cholesterol transport in mammalian cells. Cell. 175: 514–529.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naito T., Ercan B., Krshnan L., Triebl A., Koh D. H. Z., Wei F. Y., Tomizawa K., Torta F. T., Wenk M. R., and Saheki Y.. 2019. Movement of accessible plasma membrane cholesterol by the GRAMD1 lipid transfer protein complex. eLife. 8: e51401. [DOI] [PMC free article] [PubMed] [Google Scholar]