FIG 4.
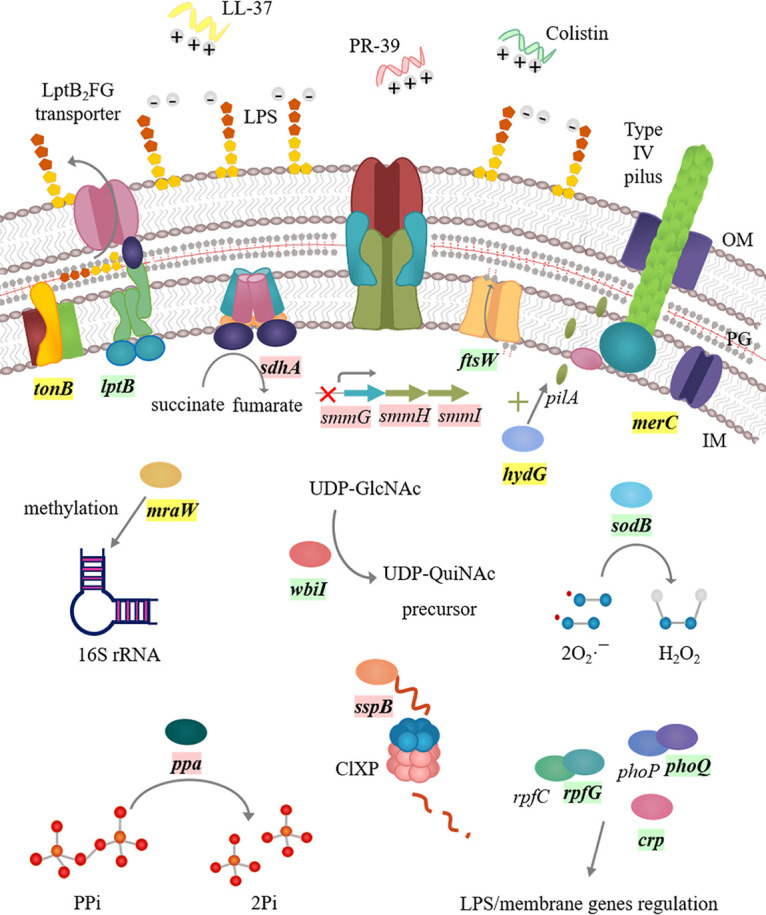
Schematic drawing of S. maltophilia acquired AMP resistance mechanisms. LL-37, PR-39 and colistin (all positively charged) interact with the negatively charged bacterial membrane to exert their action. Mutated genes are marked in bold, being specifically selected after LL-37 (yellow), PR-39 (pink), or colistin (light green) exposure. S. maltophilia can modulate the membrane charge and permeability, changing its composition and reducing the membrane negative charge (lptB, ftsW, and wbiI). The activity of proteases such as ClpXP contributes to the regulation of stress-related genes, as well as that of proteases, such as metalloproteases, that degrade AMPs. S. maltophilia can also sense and respond to AMP presence through several regulators and enzymes involved in the expression of genes that can modify the bacterial membrane and lead to resistance (phoQ, rpfG, crp, mraW, and hydG). The bacterium can act against the AMP-mediated disruption of the electron transport chain (sdhA) or the ion flows across the membrane as inorganic phosphate (Pi) (ppa). The production by AMPs of hydroxyl radicals such as colistin can be counteracted by enzyme-coding genes such as sodB. The expression of multidrug efflux pumps can lead to AMP extrusion outside the cell (smmGHI), decreasing the accumulation of the drug. IM, inner membrane; OM, outer membrane; PG, peptidoglycan; LPS, lipopolysaccharide.
