Abstract
PURPOSE
The burden of cancer is growing in low- and middle-income countries (LMICs), including sub-Saharan Africa. Ensuring the delivery of high-quality cancer care in such regions is a pressing concern. There is a need for strategies to identify meaningful and relevant quality measures that are applicable to and usable for quality measurement and improvement in resource-constrained settings.
METHODS
To identify quality measures for breast cancer care at Butaro Cancer Center of Excellence (BCCOE) in Rwanda, we used a modified Delphi process engaging two panels of experts, one with expertise in breast cancer evidence and measures used in high-income countries and one with expertise in cancer care delivery in Rwanda.
RESULTS
Our systematic review of the literature yielded no publications describing breast cancer quality measures developed in a low-income country, but it did provide 40 quality measures, which we adapted for relevance to our setting. After two surveys, one conference call, and one in-person meeting, 17 measures were identified as relevant to pathology, staging and treatment planning, surgery, chemotherapy, endocrine therapy, palliative care, and retention in care. Successes of the process included participation by a diverse set of global experts and engagement of the BCCOE community in quality measurement and improvement. Anticipated challenges include the need to continually refine these measures as resources, protocols, and measurement capacity rapidly evolve in Rwanda.
CONCLUSION
A modified Delphi process engaging both global and local expertise was a promising strategy to identify quality measures for breast cancer in Rwanda. The process and resulting measures may also be relevant for other LMIC cancer facilities. Next steps include validation of these measures in a retrospective cohort of patients with breast cancer.
INTRODUCTION
Cancer is a growing public health issue in low- and middle-income countries (LMICs), where outcomes are poor compared with those in high-income countries (HICs).1 Facilitating access to cancer care in LMICs has been increasingly prioritized by international health groups such as the WHO,2 nongovernmental organizations, and LMIC governments. As access to cancer therapy expands in limited-resource settings, systems and strategies must be in place to monitor and improve the quality of care provided. One important step toward high-quality cancer care in LMICs is the development of evidence-based treatment guidelines that are tailored to the resources of and challenges faced in low-resource settings; Breast Health Global Initiative (BHGI), the National Comprehensive Cancer Network, and others have taken important strides in developing such guidelines.3,4 In addition, to assess care quality and guide quality improvement, LMIC facilities require quality metrics tailored to their settings that consider the different care delivery challenges, treatment resources, measurement capacity, and often rapidly developing cancer care programs5,6 found in LMICs. Because breast cancer is the first- or second-most common cancer among women in most LMICs, including in sub-Saharan Africa,7 and numerous quality measures for breast cancer care exist for HICs, breast cancer care is a promising area of focus for early quality measurement efforts in LMICs.
CONTEXT
Key Objective
The objective of this work was to identify measures to assess the quality of breast cancer care at a Rwandan cancer facility, Butaro Cancer Center of Excellence (BCCOE), that were relevant to existing care delivery and resources, important for patient outcomes, actionable, and measurable using available data.
Knowledge Generated
Through a systematic review followed by a modified Delphi process engaging a panel of global cancer care quality experts and a panel of experts in cancer care delivery at BCCOE, we identified 17 measures to assess the quality of breast cancer care.
Relevance
The measures we identified and the process we undertook may be relevant to other cancer facilities in resource-constrained environments seeking to identify breast cancer care quality measures tailored to their own settings.
We sought to identify quality measures that could be used to evaluate and improve care at Butaro Cancer Center of Excellence (BCCOE) in Rwanda. Rwanda, a low-income country (LIC) of 12.5 million in East Africa, has been a regional leader in cancer control policy and programs, including with its establishment of BCCOE, Rwanda’s first public cancer facility in the country’s rural northwest. Key features of BCCOE include the provision of care largely by nononcologists using standardized context-specific cancer protocols, regular consultation with one on-site oncologist and oncology specialists based overseas, use of an oncology-specific electronic medical record, and provision of care largely free of charge to patients, with support for transport and food for the lowest-income patients.8 Breast cancer is the most common cancer treated at BCCOE, with approximately 1,800 breast cancer cases managed from 2012 to 2019. In line with expert recommendations for selection of quality measures, our goal was to identify measures that were:
Relevant to care provided at BCCOE
Potentially important for clinical outcomes (ideally based on high-quality scientific evidence)
Related to aspects of care that were thought to be variable in performance and could be influenced
Related to processes that were feasible to measure using available resources or data collection systems.9
We intended two roles for our measures: (1) retrospective application to a data set containing information on treatment from 2012 to 2016 to identify care quality gaps and their relationship to outcomes, and (2) use by the cancer center now (eg, use of the BCCOE electronic medical record) to prospectively evaluate and improve care. Because of these goals, we focused our search on process measures rather than structural or outcome measures of care quality.10
Health care quality measures are often identified through engaging health care providers and other experts in the Delphi process, which was originally developed by the RAND Corporation in the 1950s.11 The Delphi process is a systematic way to compile opinions and build consensus among a group of experts; it may be particularly valuable when evidence from the published literature is not applicable or not specific enough to apply to a given clinical setting.12 The modified Delphi technique incorporates face-to-face meetings. As we considered the process to use for BCCOE, we recognized the need for diverse expertise to develop measures that were evidence based, clinically important, and also relevant to our setting, which differs significantly from the settings in which existing quality measures and the published evidence supporting them were generated. For example, resources that are routinely used for breast cancer care in high-income settings, such as trastuzumab and radiotherapy, are not yet available for routine breast cancer care at BCCOE or in Rwanda. It was therefore important for our modified Delphi process participants to have deep expertise in BCCOE oncology protocols, available resources, care delivery model, existing quality gaps, and measurement opportunities and challenges. To capture this expertise, we engaged a panel of local experts involved in cancer care at BCCOE. However, as nononcologists, most BCCOE clinicians have less expertise in breast cancer therapy trial data and quality measures used in high-income settings. Therefore, to simultaneously identify clinical metrics likely to affect patient outcomes, we established a second panel of global experts in breast cancer care and/or quality measurement, some of whom had expertise in cancer care in LMICs. Here we describe the adapted modified Delphi process and resulting list of measures we developed, which could be adapted by other LMIC facilities.
METHODS
Systematic Literature Review and Development of Preliminary Measures List
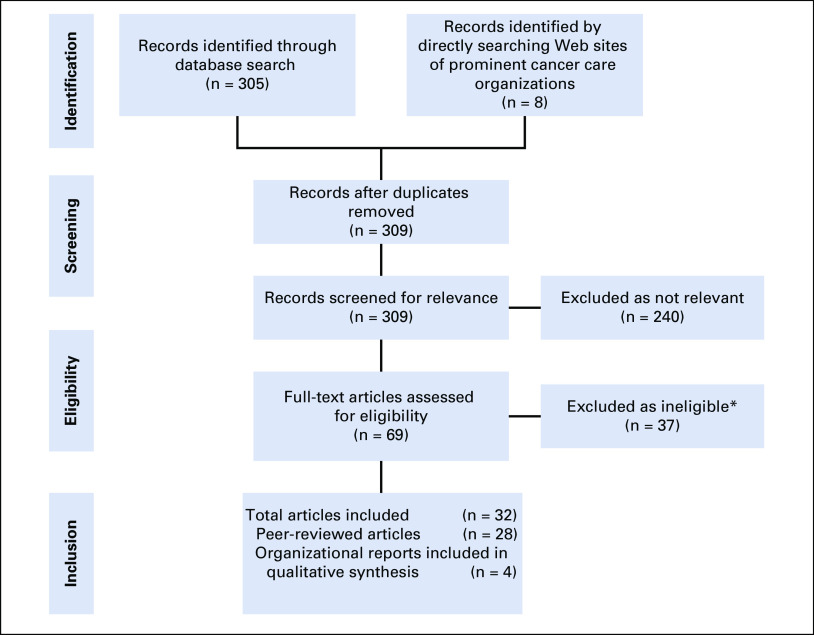
We first conducted a systematic literature review of published breast cancer quality measures, in accordance with the PRISMA guidelines13 (Fig 1). Data collected for this review came from a search on PubMed conducted on June 16, 2017, and updated on September 26, 2017 (Appendix). In addition, we manually searched the Web sites of the National Comprehensive Cancer Network,14 ASCO,15 the European Society of Breast Cancer Specialists,16 the American College of Surgeons Commission on Cancer,17 the National Accreditation Program for Breast Centers,18 the National Health Service of the United Kingdom,19 the National Health Service of Scotland,20 the National Quality Measures Clearinghouse,21 the Agency for Healthcare Research and Quality,22 and the Joint Commission.23
FIG 1.
Systematic literature search strategy and results. (*) Reasons for exclusion of measure: examined evidence for or against single quality measure (eg, re-excision lumpectomy rate, preoperative needle diagnosis, sentinel lymph node dissection); developed to assess one specific component of cancer care (eg, radiotherapy, sentinel lymph node biopsy); intended to measure quality of breast cancer screening programs; developed specifically for subpopulation of patients (eg, vulnerable elderly patients); used cancer-related indicators to assess national health care quality (eg, 5-year survival); or described application of previously published quality measures to new population.
We included all English-language articles published before September 2017 that described the development of quality measures for the diagnosis or treatment of breast cancer that were produced through at least one systematic process, either a review of scientific literature, validation with clinical data, or expert panel determination. If multiple iterations of metrics from the same organization were published, the most recent published version was included. More details on our search strategy are outlined in Figure 1 and the Appendix.
From our literature search, we extracted a comprehensive list of measures. We refined and adapted this list based on treatment modalities available at BCCOE and adapted the measures to the BCCOE setting with input from an advisory panel of global breast cancer experts consisting of one surgeon, one medical oncologist, one cancer care quality researcher, and two implementation scientists (this group did not overlap with the expert panels used in the modified Delphi process). We additionally included new measures that were particularly pertinent to LMIC settings. These included measures assessing efforts to retain patients in care, because loss to follow up and treatment noncompletion are major issues at BCCOE and in other LMICs. This resulted in a list of 40 proposed measures.
Modified Delphi Process
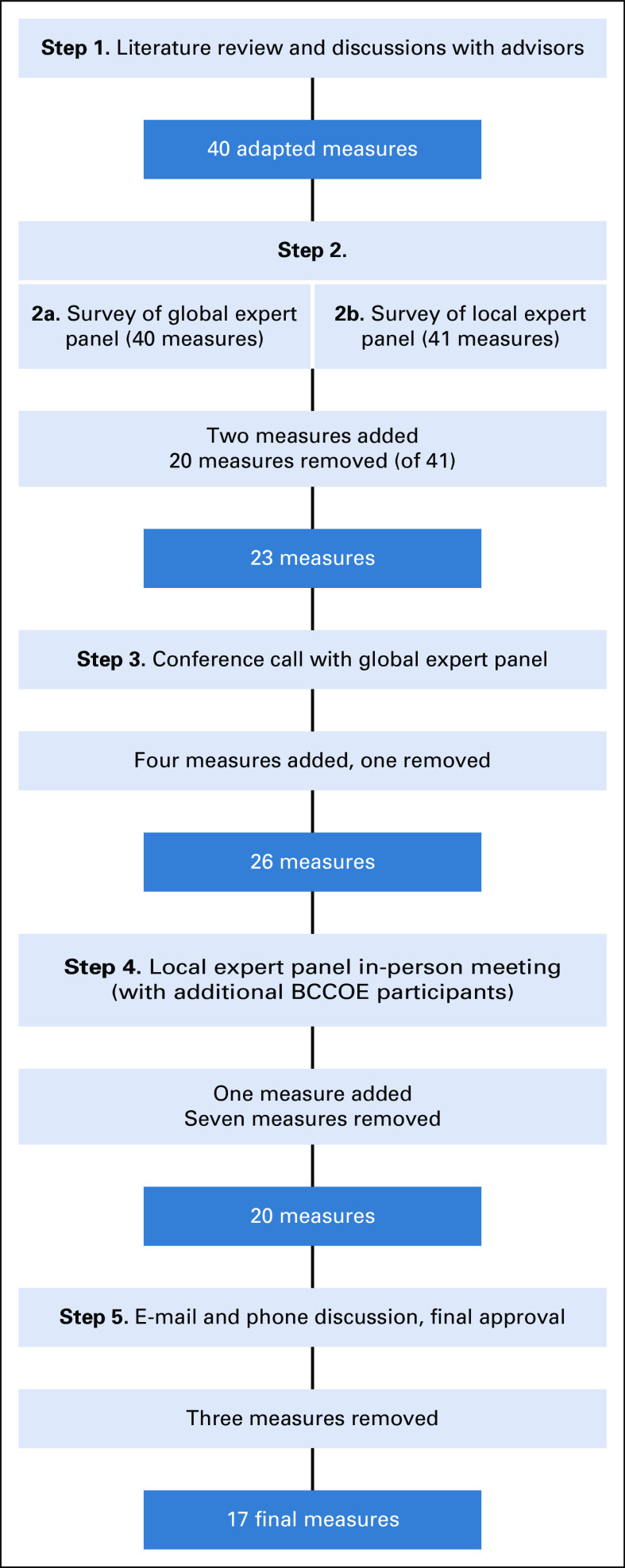
To elicit expert input regarding quality measures that were important and relevant at BCCOE, we used a modified Delphi process that we adapted further to meet our need to identify measures for an LMIC setting. The process we undertook in our modified Delphi process is outlined in Figure 2.
FIG 2.
Schematic for modified Delphi process. BCCOE, Butaro Cancer Center of Excellence.
For the global panel, after input from the advisory panel, we identified 10 experts in breast cancer pathology, medical and surgical treatment, and palliative care working in United States, the WHO, and Ghana, several of whom had spent some time at BCCOE. Seven experts (70%) responded to our invitation and agreed to participate. Those responding consisted of three surgeons, two of whom have worked at and trained other surgeons at BCCOE, one pathologist who has worked at BCCOE, and three medical oncologists, one of whom has worked at BCCOE. To this group, we sent an anonymous online survey, which asked respondents to rate 40 measures on the likelihood that concordance with each measure would improve patient outcomes.
For the local experts, we identified six staff clinicians and nonclinicians representing clinical care, program leadership, and health informatics at BCCOE. Five (83%) of these panelists completed an in-person survey, administered by the principal investigator of the study (L.E.P.). This survey included the 40 measures sent to the global experts, plus one additional measure (on multidisciplinary team care) suggested by the global experts. The survey asked experts to rank each measure on two domains: feasibility of measurement and feasibility of making changes in care delivery to improve measure concordance.
On the basis of the items with the highest rankings for both clinical relevance and feasibility, along with panelists’ suggestions, 20 measures were eliminated, two new measures were added, and some remaining measures were modified, resulting in 23 measures. We shared the survey results and refined the list of 23 quality measures with the global expert panel. We reviewed the first 23 quality measures through discussion on a conference call. At the end of the call, we administered an anonymous electronic poll where global expert panel members specifically rated the value of the remaining four measures, which had been identified previously as clinically important but requiring substantial modifications to data collection systems. We modified the measures list based on the conference call and electronic poll feedback. Four measures were added and one removed, yielding a list of 26 quality measures.
The revised list of 26 measures and survey results were then shared in person with the five BCCOE-based local experts who had participated in the in-person interviews. Additional staff joined out of interest, resulting in 10 BCCOE-based participants in the meeting, all of whom were clinical staff or clinician administrators at BCCOE. We reviewed each recommended measure in detail to discuss relevance, measurability, and variability of current practice at BCCOE.
We then communicated via e-mail and several individual phone calls with the BCCOE-based and global experts to refine the final list. To achieve consensus, we e-mailed BCCOE and global experts with a near-final list of 20 quality measures. Additional revisions and refinement continued via e-mail and individual calls with panel members, resulting in a final list of 17 approved quality measures. This research project was approved by the Partners Health Care Human Research Committee and the Rwanda National Ethics Committee.
RESULTS
Literature Review
Results of our literature search are shown in Figure 1. The literature search identified 305 articles from PubMed and eight additional publications from a manual search, of which four were duplicative in terms of measures reported. Of the 309 articles, 69 were identified as relevant. Twenty-eight peer-reviewed articles met inclusion criteria. Four additional sources of quality measures not associated with a peer-reviewed journal publication were included: the ASCO Quality Oncology Practice Initiative, American College of Surgeons Commission on Cancer, National Quality Forum Quality Positioning System, and National Health System Healthcare Improvement Scotland.
We identified 521 published quality measures from 12 coun-tries (the United States, Canada, France, Germany, Belgium, the Netherlands, Italy, Scotland, Japan, China, Taiwan, and Australia). Of the 28 identified peer-reviewed articles (Appendix), 26 (93%) originated from HICs or high-income regions, one from an upper middle-income country (China), and one from the international BHGI, which proposed qual-ity measures to assess phased implementation of breast cancer services in LMICs. No articles or resources other than the BHGI article provided suggestions for adapting quality measures to limited resources.24
Delphi Process
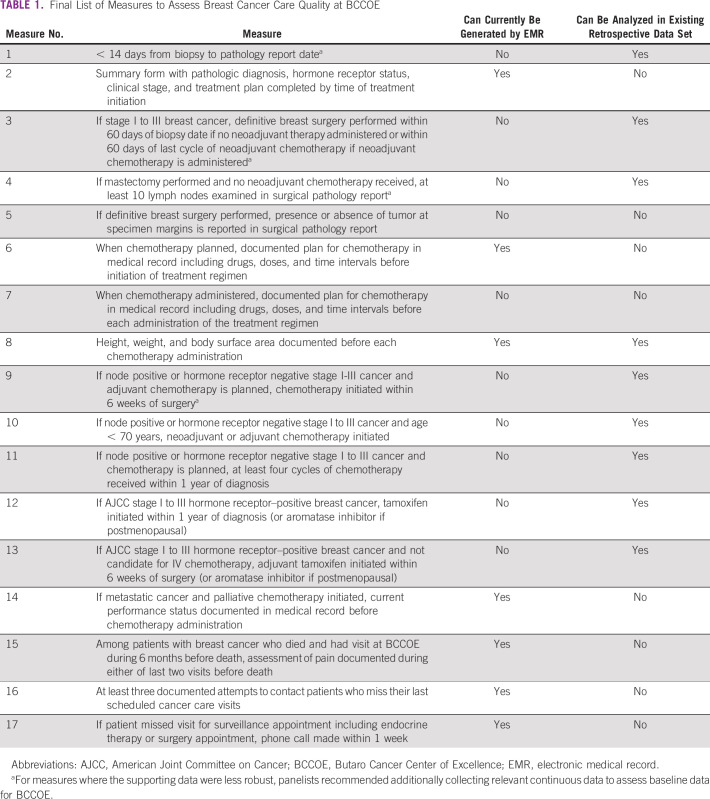
The modified Delphi rounds resulted in a final list of 17 quality measures that were determined by global and BCCOE-based experts to be clinically and contextually relevant, measurable, and feasible to have an impact through practice improvements (Table 1). Two quality measures were related to pathology (measures 1 and 5), one to staging and treatment planning (measure 2), two to surgery (measures 3 and 4), seven to chemotherapy (measures 6-11 and 14), two to endocrine therapy (measures 12 and 13), two to palliative care (measures 15 and 16), and two to retaining patients in care (measures 16 and 17). All of the final measures were adapted from measures from our literature review of measures predominantly from HICs, except for the two measures (measures 16 and 17) assessing patient contact and retention in care, which were developed specifically in response to the challenge of retaining and supporting patients at BCCOE. Of the final measures, we also determined which could be applied to existing retrospective cohort data on patients with breast cancer at BCCOE to permit validation and which could currently be extracted from the electronic medical record to assess ongoing and future care delivery (Table 1). Given the uncertainty around the optimal time intervals for some measures, such as time from neoadjuvant chemotherapy to surgery and time from surgery to adjuvant chemotherapy, and the appropriate target timeframes for BCCOE, we also include as footnotes to Table 1 four continuous measures of time intervals recommended by panelists to permit assessment of baseline values and guide modification of benchmarks. The pan-elists also recommended that data be collected to assess the number of lymph nodes identified on surgical pathology at BCCOE.
TABLE 1.
Final List of Measures to Assess Breast Cancer Care Quality at BCCOE
DISCUSSION
We undertook a systematic review of the literature and used an adapted, two-pronged, modified Delphi method to identify a set of breast cancer quality measures relevant to care at a rural Rwandan cancer facility using input from stakeholders with a diverse range of perspectives and experience. This process and its outcomes underscore key considerations for oncology care quality measure development in LMICs. First, our literature review demonstrated that there is a dearth of published cancer care quality measures that have been developed or examined in limited-resource settings. Second, to select and adapt appropriate measures, both clinical and contextual expertise were critically important and could not be provided by the same panel or set of questions. Diversity in expertise, knowledge, and experience was felt to be key in developing useful measures. Because there is only one full-time oncology specialist working at BCCOE, we engaged two panels in addressing different dimensions of the measures under consideration. Although this added logistic complexity, it ensured that we considered a wide range of relevant issues for measure development.
Some of the key considerations that influenced our selection of quality measures for a Rwandan facility were identical to core considerations guiding measure development in high-income settings, where many of the same general challenges to quality measurement exist. These include finite resources for measurement, reporting, and care, the need for clinician and administrator engagement and buy-in, and particular challenges in measuring important but complex aspects of cancer care, including patient access, equity, and experience. However, LICs face the unique challenge that published measures (largely reflecting high-income settings) may not reflect the treatment resources currently available in LIC facilities. In addition, available resources in LICs may be particularly dynamic as treatment capacity expands. Our effort generated a list of measures relevant to available resources, but this list will need continual updating as availability of different treatment modalities, such as radiotherapy, expand. In addition, measurement capability is less robust than in a high-income setting, and it is also dynamic. For example, the BCCOE electronic medical record is rapidly developing, with evolving reporting capabilities. This may increase the number of measures that can be routinely captured. Measures related to surgical care are particularly challenging to capture currently, but improved coordination between the oncology and surgical departments may mitigate this challenge and allow better assessment of surgical processes and outcomes.
Along with clinical protocols, quality measures can serve as a guide for phased priority setting and implementation of new therapies or approaches to care. The measures that we collectively identified for BCCOE helped initiate conversations about care quality, the need for systematic quality improvement programs, and priority areas to address. These discussions ultimately helped launch a new quality improvement training initiative at BCCOE.
The next step for this project will be to validate the identified measures in an existing cohort of patients with breast cancer at BCCOE. We will revise measures as needed and pilot select measures using the electronic medical record to determine the feasibility of prospective, routine measurement to inform future quality improvement projects. Ongoing review and revision will also be required as resources expand and protocols evolve.
In conclusion, promoting quality in cancer care delivery in LMICs is an increasingly pressing public health concern as the burden of cancer grows and efforts to control cancer expand. Unique challenges in providing cancer care in LMICs help shape the need for quality assessment and improvement. A modified Delphi process engaging two panels with diverse perspectives yielded valuable insights into challenges and opportunities for quality measurement at a cancer facility in rural Rwanda. Our experience provides a model for identifying meaningful, contextually relevant quality measures that can be used to inform quality improvement. Our final list of measures may also provide a valuable starting point for other cancer facilities with similar treatment protocols and available resources.
ACKNOWLEDGMENT
We thank Sharon Giordano, Jo Anne Zujewski, Rachel Freedman, and Joe-Nat Clegg-Lamptey for their guidance in measure development.
Appendix
Detailed Search Strategy
Exclusion criteria.
We searched PubMed using MeSH terms “breast neoplasms” and “healthcare quality indicator” and again using general search terms “breast cancer” and “quality indicator[s]”, “quality measure[s]”, and “quality metric[s]”.
We excluded articles examining the evidence for or against a single quality measure (eg, re-excision lumpectomy rate, preoperative needle diagnosis, and sentinel lymph node dissection), quality measures developed to assess a specific component of breast cancer care (eg, radiotherapy and sentinel lymph node biopsy), measures of quality for breast cancer screening programs, measures specific to a subpopulation of patients (eg, vulnerable elderly patients), and use of cancer care–related measures (eg, cancer screening rates and 5-year mortality) as indicators to assess national health care quality. We also excluded articles describing the application of previously published quality measures to a new patient population.
Data abstraction.
The following data were extracted from each article: quality measures, country/region of origin and sponsoring organization (if any), and selection process by which the measures were identified.
Sources for Quality Measures List Derived From Systematic Literature Review
Chapter 6: Quality improvement, in National Accreditation Program for Breast Centers Standards Manual (ed 2014). Chicago, IL, National Accreditation Program for Breast Centers, 2014, pp 73-76. https://www.facs.org/quality-programs/napbc/standards
Healthcare Improvement Scotland: Breast Cancer Clinical Quality Performance Indicators (version 3.0). http://www.healthcareimprovementscotland.org/our_work/cancer_care_improvement/cancer_qpis/quality_performance_indicators.aspx
American Society of Clinical Oncology: QOPI-related measures. https://practice.asco.org/quality-improvement/quality-programs/quality-oncology-practice-initiative/qopi-related-measures
American College of Surgeons Commission on Cancer: Breast measures. https://www.facs.org/quality-programs/cancer/ncdb/qualitymeasures
National Quality Forum: Quality Positioning System: Cancer—Breast. http://www.qualityforum.org/QPS
Anderson et al24
Barni S, Venturini M, Molino A, et al: Importance of adherence to guidelines in breast cancer clinical practice: The Italian experience (AIOM). Tumori 97:559-563, 2011
Bao H, Yang F, Wang X, et al: Developing a set of quality indicators for breast cancer care in China. Int J Qual Health Care 27:291-296, 2015
Caldarella A, Amunni G, Angiolini C, et al: Feasibility of evaluating quality cancer care using registry data and electronic health records: A population-based study. Int J Qual Health Care 24:411-418, 2012
Camps C, Albanell A, Antón A, et al: Quality indicators to assure and improve cancer care in Spain using the Delphi technique. J Natl Compr Canc Netw 14:553-558, 2016
Cheng SH, Wang CJ, Lin J-L, et al: Adherence to quality indicators and survival in patients with breast cancer. Med Care 47:217-225, 2009
Chin-Lenn L, Craighead P, Bryant HE, et al: Quality indicators for ductal carcinoma in situ (DCIS) of the breast: Development using a multidisciplinary Delphi process and its use in monitoring population-based treatment. J Surg Oncol 108:348-351, 2013
Chung K-P, Lai M-S, Cheng SH, et al: Organization-based performance measures of cancer care quality: Core measure development for breast cancer in Taiwan. Eur J Cancer Care (Engl) 17:5-18, 2008
Clifford EJ, De Vol EB, Pockaj BA, et al: Early results from a novel quality outcomes program: The American Society of Breast Surgeons’ mastery of breast surgery. Ann Surg Oncol 17:233-241, 2010 (suppl 3)
Cornfeld MJ, Jadwin A, O’Grady P, et al: Feasibility of assessing quality in medical oncology practices. Cancer Pract 9:231-235, 2001
Crowe P: Improving surgical outcomes for patients with cancer: An Australian perspective. J Surg Oncol 99:478-480, 2009
Del Turco MR, Ponti A, Bick U, et al: Quality indicators in breast cancer care. Eur J Cancer 46:2344-2356, 2010
Desch CE, McNiff KK, Schneider EC, et al: American Society of Clinical Oncology/National Comprehensive Cancer Network quality measures. J Clin Oncol 26:3631-3637, 2008
Ferrua M, Couralet M, Nitenberg G, et al: Development and feasibility of a set of quality indicators relative to the timeliness and organisation of care for new breast cancer patients undergoing surgery. BMC Health Serv Res 12:167, 2012
Fessele K, Yendro S, Mallory G: Setting the bar: Developing quality measures and education programs to define evidence-based, patient-centered, high-quality care. Clin J Oncol Nurs 18:7-11, 2014 (suppl)
Grunfeld E, Lethbridge L, Dewar R, et al: Towards using administrative databases to measure population-based indicators of quality of end-of-life care: Testing the methodology. Palliat Med 20:769-777, 2006
Grunfeld E, Urquhart R, Mykhalovskiy E, et al: Toward population-based indicators of quality end-of-life care: Testing stakeholder agreement. Cancer 112:2301-2308, 2008
Iwamoto M, Nakamura F, Higashi T: Monitoring and evaluating the quality of cancer care in Japan using administrative claims data. Cancer Sci 107:68-75, 2016
Khare SR, Batist G, Bartlett G: Identification of performance indicators across a network of clinical cancer programs. Curr Oncol 23:81-90, 2016
Kowalski C, Ferencz J, Brucker SY, et al: Quality of care in breast cancer centers: Results of benchmarking by the German Cancer Society and German Society for Breast Diseases. Breast 24:118-123, 2015
Krzyzanowska MK, Barbera L, Elit L, et al: Identifying population-level indicators to measure the quality of cancer care for women. Int J Qual Health Care 23:554-564, 2011
Laronga C, Gray JE, Siegel EM, et al: Florida initiative for quality cancer care: Improvements in breast cancer quality indicators during a 3-year interval. J Am Coll Surg 219:638-645.e1, 2014
Lovrics P, Hodgson N, O’Brien MA, et al: The implementation of a surgeon-directed quality improvement strategy in breast cancer surgery. Am J Surg 208:50-57, 2014
Mano MP, Ponti A, Tomatis M, et al: Audit system on quality of breast cancer diagnosis and treatment (QT): Results of quality indicators on screen-detected lesions in Italy, 2007.” Epidemiol Prev 34:81-88, 2010 (suppl 4)
Mukai H, Higashi T, Sasaki M, et al: Quality evaluation of medical care for breast cancer in Japan. Int J Qual Health Care 28:110-113, 2016
Sacerdote C, Bordon R, Pitarella S, et al: Compliance with clinical practice guidelines for breast cancer treatment: A population-based study of quality-of-care indicators in Italy.” BMC Health Serv Res 13:28, 2013
Stordeur S, Vrijens F, Devriese S, et al: Developing and measuring a set of process and outcome indicators for breast cancer. Breast 21:253-260, 2012
van Bommel ACM, Spronk PER, Vrancken Peeters M-JTFD, et al: Clinical auditing as an instrument for quality improvement in breast cancer care in the Netherlands: The national NABON breast cancer audit. J Surg Oncol 115:243-249, 2017
Winchester DP: The National Accreditation Program for Breast Centers: Quality improvement through standard setting. Surg Oncol Clin N Am 20:581-586, 2011
PRIOR PRESENTATION
Presented in part at the Breast Health Global Initiative Global Summit, Seattle, WA, October 15-17, 2018.
SUPPORT
Supported by National Cancer Institute K07 Career Development Award No. 1K07CA215819-01A1 (L.E.P.).
AUTHOR CONTRIBUTIONS
Conception and design: Lydia E. Pace, Lauren E. Schleimer, Cyprien Shyirambere, Jean Marie Vianney Dusengimana, Francois Regis Uwizeye, Mary Chamberlin, Lawrence N. Shulman, Susan Troyan, Catherine Duggan, Daniel S. O’Neil, Deogratias Ruhangaza, John Butonzi, Tharcisse Mpunga, Nancy L. Keating
Financial support: Lydia E. Pace, Lawrence N. Shulman
Administrative support: Lydia E. Pace, Cyprien Shyirambere, Jean Marie Vianney Dusengimana, Lawrence N. Shulman, Eugene Nkusi
Provision of study material or patients: Lydia E. Pace, Jean Marie Vianney Dusengimana, Eugene Nkusi
Collection and assembly of data: Lydia E. Pace, Lauren E. Schleimer, André Ilbawi, Jean Marie Vianney Dusengimana, Jean Bosco Bigirimana, Francois Regis Uwizeye, Mary Chamberlin, Susan Troyan, Cam Nguyen, Olivier Habimana, Nicaise Nsabimana, John Butonzi, Eugene Nkusi
Data analysis and interpretation: Lydia E. Pace, Lauren E. Schleimer, Cyprien Shyirambere, André Ilbawi, Jean Marie Vianney Dusengimana, Francois Regis Uwizeye, Mary Chamberlin, Yeonsoo Sara Lee, Lawrence N. Shulman, Susan Troyan, Benjamin O. Anderson, Allison Dvaladze, Jane Brock, John Butonzi, Eugene Nkusi, Tharcisse Mpunga, Nancy L. Keating
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lydia E. Pace
Stock and Other Ownership Interests: Firefly Health, Becton Dickinson, Mettler Toledo, Roche, Amgen
Mary Chamberlin
Consulting or Advisory Role: Genomic Health International
Research Funding: Archer Biosciences (Inst)
Lawrence N. Shulman
Research Funding: Celgene
Benjamin O. Anderson
Consulting or Advisory Role: Allergan
Daniel S. O’Neil
Honoraria: Ipsen
Allison Dvaladze
Travel, Accommodations, Expenses: Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.3322/caac.21492. Bray F, Ferlay J, Soerjomataram I, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [Erratum: CA Cancer J Clin 70:313, 2020] [DOI] [PubMed] [Google Scholar]
- 2. doi: 10.2471/BLT.15.163998. Robertson J, Barr R, Shulman LN, et al: Essential medicines for cancer: WHO recommendations and national priorities. Bull World Health Organ 94:735-742, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network: NCCN Harmonized Guidelines for Sub-Saharan Africa. https://www.nccn.org/harmonized.
- 4.Harford J, Azavedo E, Fischietto M. Guideline implementation for breast healthcare in low- and middle-income countries: Breast healthcare program resource allocation. Cancer. 2008;113(suppl):2282–2296. doi: 10.1002/cncr.23841. [DOI] [PubMed] [Google Scholar]
- 5.Pace LE, Keating NL. The role of quality measures in improving breast cancer care in low-income countries. Curr Breast Cancer Rep. 2018;10:196–201. [Google Scholar]
- 6.Kerr DJ. Should we adapt existing quality systems for use in low- and middle-income countries? J Oncol Pract. 2015;11:370–371. doi: 10.1200/JOP.2015.006577. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization: GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- 8.Tapela NM, Mpunga T, Hedt-Gauthier B, et al. Pursuing equity in cancer care: Implementation, challenges and preliminary findings of a public cancer referral center in rural Rwanda. BMC Cancer. 2016;16:237. doi: 10.1186/s12885-016-2256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin HR, Pronovost P, Diette GB. From a process of care to a measure: The development and testing of a quality indicator. Int J Qual Health Care. 2001;13:489–496. doi: 10.1093/intqhc/13.6.489. [DOI] [PubMed] [Google Scholar]
- 10.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 11. Linstone HA, Turoff M (eds): The Delphi Method: Techniques and Applications. Boston, MA, Addison-Wesley, 1975. [Google Scholar]
- 12.Boulkedid R, Abdoul H, Loustau M, et al. Using and reporting the Delphi method for selecting healthcare quality indicators: A systematic review. PLoS One. 2011;6:e20476–e20476. doi: 10.1371/journal.pone.0020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network http://www.nccn.org/
- 15.American Society of Clinical Oncology https://www.asco.org/
- 16.European Society of Breast Cancer Specialists https://www.eusoma.org/
- 17.American College of Surgeons Commission on Cancer https://www.facs.org/quality-programs/cancer/coc
- 18.National Accreditation Program for Breast Centers https://www.facs.org/quality-programs/napbc
- 19.National Health Service United Kingdom https://www.nhs.uk/
- 20.National Health Service Scotland https://www.scot.nhs.uk/
- 21.National Quality Measures Clearinghouse doi: 10.1080/15360280802537332. http://www.qualitymeasures.ahrq.gov/ [DOI] [PubMed]
- 22.Agency for Healthcare Research and Quality doi: 10.1080/15360280802537332. https://www.ahrq.gov [DOI] [PubMed]
- 23.Joint Commission https://www.jointcommission.org/
- 24.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: Overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113(suppl):2221–2243. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]