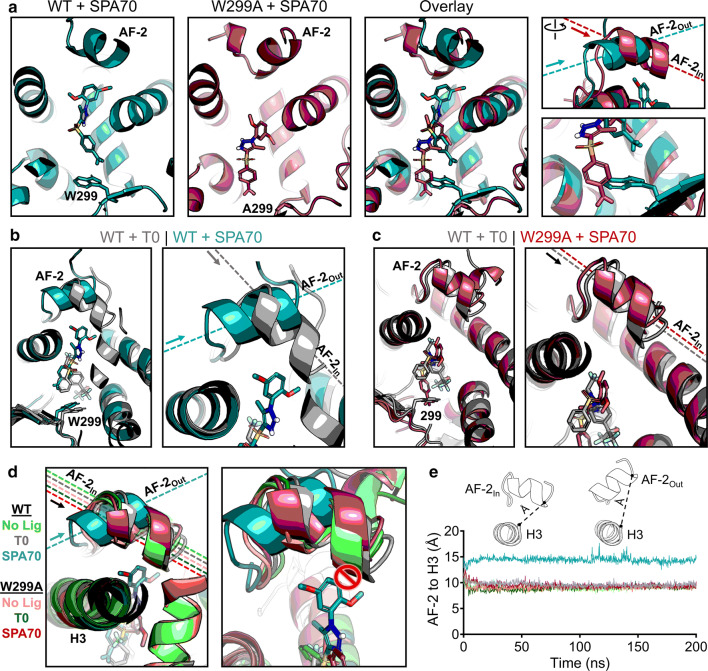
Fig. 5.
Structural mechanism of SPA70 antagonist-to-agonist switch conferred by the W299A mutation. Molecular dynamics simulations were performed for WT and W299A PXR LBD in the presence of no ligand, T0, or SPA70. a Individual and superimposed images of the ligand-binding pocket are shown for SPA70-bound WT (teal) and W299A (raspberry) PXR LBD; the right panels focus on the AF-2 helix and W/A299. The dashed lines indicate the orientation of each AF-2 helix; arrows point from the N to the C terminus of the helix. The AF-2 is labeled “In” or “Out” in reference to the proximity of the C terminus to the ligand-binding pocket. b Overlaid images of T0-bound WT (gray) and SPA70-bound WT (teal) PXR LBD. c Overlaid images of T0-bound WT (gray) and SPA70-bound W299A (raspberry) PXR LBD. d WT or W299A simulations without ligand, with T0, and with SPA70 are overlaid. Left panel: the AF-2 is in the “In” position for all simulations except SPA70-bound WT. Right panel: The AF-2 cannot adopt the “In” position in WT PXR LBD when SPA70 is present because the helix would clash with the ligand; therefore, the helix is held in the “Out” orientation. e Quantification of the “In” vs. “Out” AF-2 orientations between simulations. The distance from the AF-2 residue F429 to the Helix 3 residue F251 was measured for the duration of each simulation (using α-carbon atoms for measurements)